- Home
- »
- Medical Devices
- »
-
Hemodynamic Monitoring Devices Market Size Report, 2030GVR Report cover
![Hemodynamic Monitoring Devices Market Size, Share & Trends Report]()
Hemodynamic Monitoring Devices Market Size, Share & Trends Analysis Report, By System Type (Invasive, Minimally Invasive, Non-Invasive), By Product (Disposables, Monitors), By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: 978-1-68038-876-3
- Number of Pages: 100
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
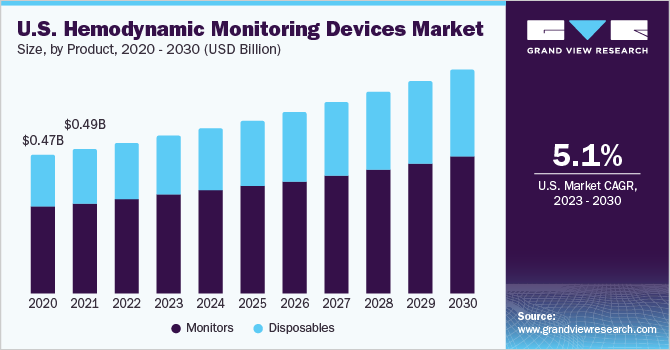
The global hemodynamic monitoring devices market was valued at USD 1.47 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 5.2% from 2023 to 2030. Hemodynamic monitoring devices screen heart function and blood flow, which aids in assessing a patient’s cardiovascular status and guiding treatment decisions. The market is expected to witness growth due to several factors, such as the increasing prevalence of cardiovascular, chronic obstructive pulmonary (COPD), and respiratory diseases, the development of novel non-invasive & minimally invasive systems, and rapid advancement in surgical procedure techniques. According to American Lung Association, the prevalence of COPD in the U.S. was estimated at 6.2% of the total population.
The pandemic had a positive impact on the hemodynamic monitoring devices market. COVID-19-induced complications, such as sepsis, low blood oxygen levels, and others requiring intensive care, increased the demand for hemodynamic devices. According to NCBI, pulmonary artery catheters (PACS) demonstrated empirical performance, which maximized monitoring capabilities and reduced healthcare expenditure in hospitals. Similarly, Deltex Medical stated that esophageal Doppler monitoring provided real-time cardiac output (CO) from the patient’s aorta, a key marker for healthcare professionals.
The development of novel techniques for hemodynamic monitoring is expected to drive market growth. Newly launched devices are expected to improve the management of a cardiovascular patient under postoperative care & anesthesia. Such devices provide accurate measurements that can detect any form of hemodynamic alteration, inform the cause of alteration, and optimize the therapeutic intervention for the patient. The transition from static to dynamic variable monitoring and the development of minimally invasive monitoring techniques is expected to drive market growth during the forecast period. For instance, in March 2021, Koninklijke Philips N.V. launched the IntelliVue X3 system. The system provides advanced hemodynamic measurements and improves the clinical focus during different procedures.
The growing prevalence of cardiovascular, respiratory, and chronic obstructive pulmonary diseases is expected to boost hemodynamic monitoring devices market. According to American Heart Association, the age-adjusted prevalence of cardiovascular disease (CVD) was 7,354.1 per 100,000 population across the globe in the year 2020. Moreover, a similar source stated that CVD mortality was 239.8 per 100,000 population. Furthermore, the increasing prevalence of respiratory diseases & development of novel technologies boosts the demand for hemodynamic monitoring devices, as these devices aid in respiration monitoring during treatment. For instance, in May 2023, Inspira Technologies OXY B.H.N. Ltd. announced to develop VORTX, a respiration technology expected to improve hemodynamic performance and reduce oxygenator failures.
System Type Insights
Based on system type, the hemodynamic monitoring device market is segmented into invasive, minimally invasive, and non-invasive. Non-invasive dominated the market with a revenue share of 55.1% in 2022. The constant progress in noninvasive continuous blood pressure measurement has been one of the areas of recent advancement in the field of blood pressure monitoring. These tools make it possible to gauge the patient's condition and measure blood pressure in real time.
The non-invasive segment is estimated to be the fastest-growing, with a CAGR of 5.6% during the forecast period. Non-invasive hemodynamic devices are used in different clinical settings, such as outpatient clinics, general wards, and homecare, to monitor patients’ cardiovascular parameters. In addition, these devices are commonly used by patients who are not critically ill but require continuous assessment. Also, there are continuous advancements which is boosting the market growth. For instance, in October 2020, Getinge launched NICCI, an advanced non-invasive monitoring solution to reduce the complications associated with low blood pressure.
Product Insights
The monitors dominated the revenue share in 2022 and accounted for 62.7% in 2022 of the hemodynamic monitoring devices market. Monitors are the most crucial part of the monitoring system as they display all the parameters that are under assessment by the healthcare professionals. Also, rising investment by major manufacturers to develop patient-centric care and bring technologically advanced product in the market is driving the hemodynamic monitors demand.
The disposables segment is estimated to be the fastest-growing, with a CAGR of 5.6% during the forecast period. The growth can be attributed to the increasing collaboration in the marketplace to improve patient monitoring. For instance, in June 2023, Mindray Medical and Edward Lifesciences Corporation collaborated to integrate the Edwards FloTrac sensor in Mindray’s hemodynamic monitor- BeneVision N. The product is expected to launch in the European market, post-market acceptance in China.
End-use Insights
Based on end-use, the hemodynamic monitoring devices market is segmented into hospitals, catheterization labs, ambulatory surgical centers, and others. Hospitals dominated the market with a revenue share of 42.6% in 2022. The share is due to the hospital's ability to manage high-acuity patients, such as those suffering from heart failure, sepsis, and shock. According to NCBI, an estimated 1.7 million patients suffer from sepsis annually in the U.S. In addition, hospitals perform invasive surgeries, which necessitates the demand for accurate hemodynamic monitoring devices.
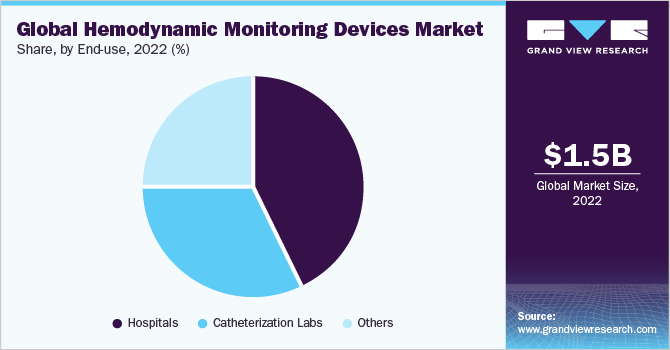
Moreover, catheterization labs are expected to witness significant growth opportunities during the forecast period. These labs provide a range of invasive surgical procedures such as coronary angiography, percutaneous coronary interventions (angioplasty and stent placement), and others that necessitates the demand for hemodynamic monitoring devices. Moreover, strategic initiatives such as expansion is expected to boost the segment growth. For instance, in July 2023, Mount Sinai Queens announced to open a new catheterization lab to provide comprehensive care to patients suffering from heart ailments.
Regional Insights
North America dominated the global market and accounted for 41.1% of the total market share in 2022. The share is attributed to the advanced healthcare infrastructure, high prevalence of cardiovascular diseases, rapid technological advancements, high healthcare expenditure, and a growing geriatric population. According to Rural Health Information Hub, the geriatric population in the U.S. is expected to increase by 18 million between 2020 - 2030. Moreover, a similar source stated that 90% of adults over 65 develop one or more chronic conditions.
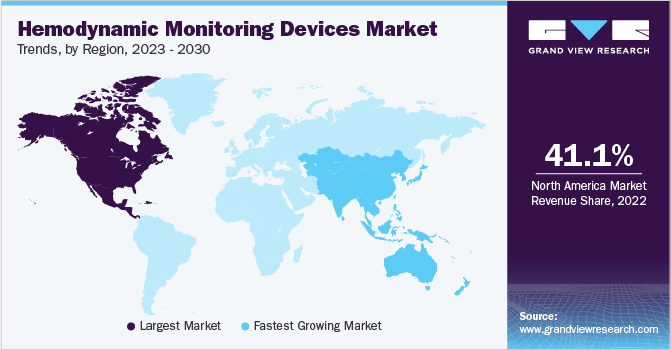
Asia-Pacific is expected to witness the fastest CAGR of 5.9% during the forecast period. The growth can be attributed to the increasing product approvals in countries such as China and Japan. For instance, in January 2022, Medtronic received approval from National Medical Products Association (NMPA) for Evolut PRO TAVR System. The system aids in treating severe aortic stenosis while providing full hemodynamic performance.
Key Companies & Market Share Insights
The market is highly competitive, with a large number of manufacturers, the market players are focusing on various strategic initiatives such as new product launches, geographical expansion, mergers and acquisitions, collaboration, product upgradation, and partnerships. For instance, in July 2022, Caretaker Medical received U.S. FDA approval to add four new parameters in its VitalStream wireless blood pressure and hemodynamic platform. The four parameters included stroke volume, cardiac output, heart rate variability, and left ventricular ejection time. The following are some of the key manufacturers in the global hemodynamic monitoring devices market:
-
Edwards Lifesciences Corporation
-
GE HealthCare
-
Baxter
-
ICU Medical
-
Koninklijke Philips N.V.
-
Sramek BioDynamics, Inc.
-
OsypkaCardiotek GmbH
-
Deltex Medical Group
-
Masimo
Hemodynamic Monitoring Devices Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 1.53 billion
Revenue forecast in 2030
USD 2.18 billion
Growth rate
CAGR of 5.2% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
September 2023
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
System type, product, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; U.K.; France; Italy; Spain; Norway; Denmark; Sweden; China; Japan; India; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Edwards Lifesciences Corporation; GE HealthCare; Baxter; ICU Medical; Koninklijke Philips N.V.; Deltex Medical Group; Sramek BioDynamics, Inc.; OsypkaCardiotek GmbH; Masimo
Customization scope
Free report customization (equivalent up to 8 analyst's working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Hemodynamic Monitoring Devices Market Report Segmentation
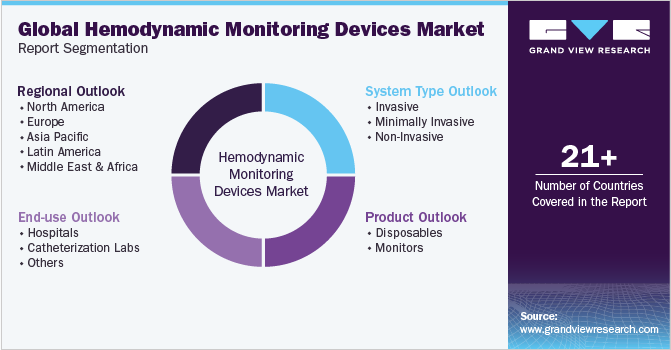
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global hemodynamic monitoring devices market report based on system type, product, end-use and region:
-
System Type Outlook (Revenue in USD Billion, 2018 - 2030)
-
Invasive
-
Minimally Invasive
-
Non-Invasive
-
-
Product Outlook (Revenue in USD Billion, 2018 - 2030)
-
Disposables
-
Monitors
-
-
End-use Outlook (Revenue in USD Billion, 2018 - 2030)
-
Hospitals
-
Catheterization Labs
-
Others
-
-
Regional Outlook (Revenue in USD Billion 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Norway
-
Denmark
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global hemodynamic monitoring devices market size was estimated at USD 1.47 billion in 2022 and is expected to reach USD 1.53 billion in 2023.
b. The global hemodynamic monitoring devices market is expected to witness moderate growth from 2023 to 2030 to reach USD 2.18 billion by 2030
b. North America dominated the hemodynamic monitoring devices market with a share of about 41.1% in 2022. This is attributable to increasing demand for minimally invasive technologies and new product launches
b. Some key players operating in the hemodynamic monitoring devices market include Edward Lifesciences, Masimo Deltex Medical, Philips Medical, GE Healthcare, ICU Medical, Sramek BioDynamics, Inc., OsypkaCardiotek GmbH
b. Key factors that are driving the market growth include increasing demand for non-invasive monitoring techniques coupled with the rising prevalence of chronic illness
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





