- Home
- »
- Medical Devices
- »
-
In Vivo CRO Market Size, Share And Trends Report, 2030GVR Report cover
![In Vivo CRO Market Size, Share & Trends Report]()
In Vivo CRO Market Size, Share & Trends Analysis Report By Model Type (Rodent Based, Non-rodent Based), By Modality (Small Molecule, Large Molecule), By Indication, By GLP Type (Non GLP, GLP Toxicology), By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-1-68038-011-8
- Number of Pages: 257
- Format: Electronic (PDF)
- Historical Range: 2018 - 2023
- Industry: Healthcare
In Vivo CRO Market Size & Trends
The global in vivo CRO market size was estimated at USD 4.59 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 8.1% from 2024 to 2030. The increasing burden of cancer patients, growing in vivo pharmacology studies, and the emerging number of pharma and biotechnology companies focusing on researching and developing novel therapeutics are expected to drive market growth during the forecast period.
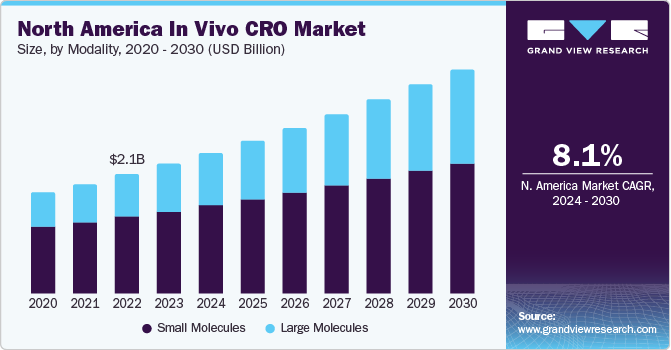
In addition, in vivo CROs are specifically backed by the rising number of CROs, research and development activities, and preclinical studies, which have led to a rise in market growth. Furthermore, growing investments across in vivo gene-modified cell therapies are expected to boost market growth. For instance, in November 2023, the CCR researchers announced the development of a new technology to support T-cell therapies to function better against solid tumors utilizing mice models with pediatric and adult cancers.
The COVID-19 pandemic did not positively impact the clinical trials and in vivo CRO industries. However, the effect of the pandemic was comparatively moderate across the in vivo CRO industry, as several contract research organizations continuously focused on the R&D of COVID-19 treatment therapeutics, thereby providing in vivo preclinical services for the same. The integrity of over 330,000 clinical trials registered on clinicaltrials.gov remains vulnerable as the coronavirus outbreak spreads worldwide. Furthermore, as of March 2020, at least 18 pharmaceutical or biotechnology companies have reported a disturbance to a clinical trial due to this pandemic. However, post-pandemic, there was a growing demand for novel technologies and an increasing need for patient-friendly drugs, which has led to improvement in the pipeline of pharmaceuticals due to stringent regulations by regulatory agencies such as the FDA and EMA, pharmaceutical & biopharmaceutical companies outsourcing their medical affairs to streamline the regulatory process. Thus, this is expected to impact market growth positively in the coming years.
Moreover, rising complexity with respect to new drug development has fueled the market’s growth. Manufacturing high-quality and safe drugs for patient care is a major concern for drug manufacturers. Moreover, the regulations related to drugs and biologics are vast and are complicated by legal technicalities as it is a rapidly growing field. The complexity of the drug development process has considerably grown in recent years. Pharmaceutical and biotechnology companies are outsourcing their complex in vivo activities to CROs and focusing on business development.
In addition, the rising demand for advanced products, such as rare disease therapies and anti-cancer medicines, is one of the major factors supporting the growth of the market. For instance, organizations such as the European Society of Medical Oncology (ESMO) and Latin American Society of Clinical Oncology (SLACOM) engage in collaborative work with the Peruvian Cooperative Oncology Group. These favorable initiatives are anticipated to generate lucrative revenue over the forecast period. Furthermore, in October 2023, Coeptis Therapeutics Holdings, Inc. announced research demonstrating the possibility of the SNAP-CAR T-cell platform to target numerous antigens. The research involved SNAP-CAR to demonstrate the technology's adaptable antigen-targeting abilities in vivo and in-vitro in xenograft models of human tumors.
Furthermore, a growing pipeline of cell and gene therapies is anticipated to increase the opportunity for market growth. Advancements in manufacturing, including improved accuracy, manufacturing, and control regulations, have led to tremendous growth in the past few years. In November 2023, the National Cancer Institute (NC) researchers designed a way to potentially improve the efficacy of T cell-based immunotherapy therapies, such as CAR T-cell therapy, to treat solid tumors. In animal-based studies, improved T-cell therapies efficiently treated neuroblastoma and cervical cancer. This is expected to increase the demand of the market, thereby contributing to market profits.
Market Concentration & Characteristics
Market growth stage is medium, and pace of the market growth is accelerating. The in vivo CRO sector is characterized by a high degree of innovation owing to the rising need for drug discovery, increasing chronic diseases, and rapid technological advancements. The market is also driven by the rising incidence of cancers, increased demand for innovative products, including CRISPR gene editing and advanced imaging systems. In addition, the need for increased efficiency in preclinical studies has led to the incorporation of automation, data analytics, and artificial intelligence.
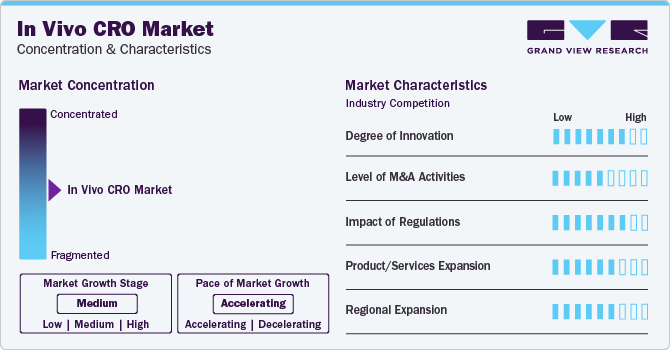
The market is also characterized by the leading players' moderate merger and acquisition (M&A) activity. These activities enable companies to expand service offerings, achieve operational efficiency, strengthen market presence, and gain access to new markets. It benefits the companies by enhancing their expertise, achieving cost savings, and competing more effectively in the competitive landscape of preclinical research services.
The market is also subject to increasing regulatory scrutiny. Regulations profoundly impact the market, influencing ethical standards, animal welfare considerations, and the overall conduct of preclinical research. Stringent compliance with regulatory requirements, including Good Laboratory Practice (GLP) guidelines, is crucial for the credibility and acceptance of in vivo study outcomes. The dynamic regulatory landscape also drives innovation within the market, encouraging CROs to adapt to evolving standards, such as increased emphasis on transparency and reproducibility, to maintain compliance and competitiveness.
The market is characterized by accelerating product/service expansion. CROs respond to client needs by diversifying their portfolios with advanced in vivo models, specialized assays, and innovative technologies. The purpose of personalized medicine and the outsourcing trend in the pharmaceutical industry further drive the need for comprehensive, one-stop-shop solutions.
It is driven by the global need for drug development, increased outsourcing, and the desire to offer localized services. CROs are establishing a presence in key regions to serve diverse patient populations, understand local regulatory requirements, and enhance collaboration with pharmaceutical and biotech clients. In addition, this expansion strategy allows CROs to enter emerging markets, capitalize on regional strengths, and provide comprehensive preclinical research solutions globally.
Model Type Insights
In 2023, the rodent based segment dominated the market with a revenue share of 81.8%. The rodent based segment is further sub-segmented to rat models, mice models, and others. Rodents are commonly used as models in medical research due to their similarities to humans in behavior, biology, and genetics. They are extensively used in medical trials and studies, making them the most prevalent species in such research. According to the Foundation for Biomedical Research (FBR), rodents account for around 95% of all laboratory animals. Also, rodents born without immune systems, such as Severe Combined Immune Deficiency (SCID) mice, can be models for malignant and normal human tissue research. Moreover, according to the Koshland Science Museum, rats share approximately 90% of genes with humans. Thereby, increased adoption of rodents-based models across in-vivo studies has fueled the demand for outsourced in vivo services, thus contributing to the market growth.
On the other hand, the non-rodent based segment is expected to witness a significant CAGR over the forecast period. The non-rodent segment is among the mostly used animal models for research and offers several advantages, such as genetic homology to humans, metabolism, body weight, sequential sampling, life span, and organ structure. However, breeding, housing, and handling non-rodent animals can be challenging. Furthermore, according to the UK Research and Innovation (UKRI), as of May 2023, 31% of the Medical Research Council (MRC)-funded research grants used animals, of which 78% were mice, 10% rats, 4% pigs, and 8% other animals.
Modality Insights
In 2023, the small molecules segment held the largest revenue share. The small molecules are studied across small molecule APIs, highly potent active pharmaceutical ingredients (HPAPI), etc. The number of small molecule drug candidates is far more extensive than large molecules. Furthermore, these molecules support in preclinical & clinical development of drug candidates with extensive academic research for novel targets. In August 2023, Design Therapeutics, Inc., specializing in clinical-stage biotechnology, announced business updates and upcoming results of new GeneTAC small molecules.Thereby, different advantages of small molecules and constant investment across small molecules drug development are some of the factors supporting the segment growth.
The large molecules segment is anticipated to grow at the fastest CAGR during the forecast period. The segment is further sub-segmented in Cell & Gene Therapy (CAR T-cell therapies, CAR-NK cell therapy, TCR-T cell therapy, Other (Includes- TCR-NK, CAR-M, and TAC-T)), RNA Therapy, and Others. In vivo CROs play a crucial role in the investigation of large molecules in preclinical and clinical trials. These organizations have special skills and expertise in handling and testing large molecules in appropriate animal models. Safety and toxicity studies, pharmacokinetic (pk) studies, efficacy studies, and immunogenicity studies are some of the common services offered by CROs. Therefore, these factors favor the growth of the segment.
GLP Type Insights
The GLP toxicology segment dominated the market with the largest revenue share in 2023. Clinical studies such as safety pharmacology, genotoxicity, and repeated dose toxicity are mandatory for safe exposure to humans and must be performed as per the GLP standards. These studies are required to be conducted before the IND application. After IND approval, other GLP experiments to evaluate developmental and reproductive toxicity, chronic toxicity, genotoxicity, and carcinogenicity must be conducted during the clinical phase of development. For the evaluation of safety studies, compliance with GLP standards is mandatory.
Increasing clinical trials for oncology studies are expected to boost market growth. For instance, in January 2023, Qualigen Therapeutics initiated the GLP toxicology studies of its QN-302, an oncology program. The research would be conducted by WuXi AppTec. GLP toxicology tests are a crucial part of QN-302's investigational new drug (IND) filing package, which was anticipated to happen in the first half of 2023. After the IND is approved by the U.S. FDA, human clinical trials will be conducted. Hence, the growing number of clinical research for novel therapeutics is one of the major factors contributing to the segment’s growth.
On the other hand, the non GLP segment is anticipated to witness a significant CAGR during the analysis period. Non-GLP toxicology studies are performed with the same high standards but need not adhere to all GLP guidelines. These toxicological studies are performed in accordance with high standards of quality that ensure the validity and accuracy of the study data required to assess test object or item. In addition, the drug development process involves clinical and nonclinical studies. These nonclinical studies are performed using various rules, including animal studies, which mostly comply with GLP regulations. Thereby, non-GLP toxicology is expected to witness growth at stable growth rate over the estimated time period.
Indication Insights
The oncology segment held the largest market share in 2023. In oncology, mouse models are considered an ideal model for human cancer research across in vivo studies due to the relatively similar physiological and genomic characteristics of tumors in humans and mice. Mice have several similar molecular, cellular, and anatomical characteristics to humans. These characteristics are known to have critical functions & properties in cancer. Mice genes, particularly RNI-like genes, exhibit more than 80% similarity to their human counterparts, enabling researchers to use mice as experimentally tractable models to investigate treatment responses and basic mechanisms of cancer development. The most widely used and affordable traditional models for tumor studies are immunodeficient and immunocompetent mice models with xenografted and syngeneic tumors transplanted orthotopically or subcutaneously. Hence, the aforementioned factors contribute to the lion’s share of the segment during the analysis period.
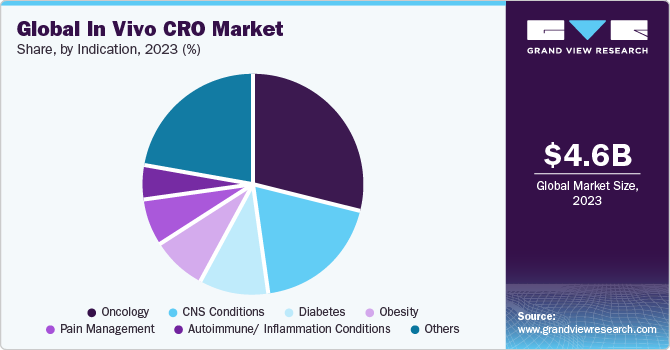
The CNS conditions segment is expected to register the fastest growth over the forecast period. Epilepsy, Parkinson’s disease (PD), Huntington’s disease (HD), stroke, and Traumatic Brain Injury (TBI), among others, are some of the major CNS disorders. In vivo CROs play a key role in CNS research by offering specialized services and understanding for conducting preclinical investigations in animal models. Furthermore, at present, several pipeline of CNS drugs are available in the market. However, the discovery & development of new drugs possessing the required efficacy & tolerability is still a major concern.
Regional Insights
North America dominated the market with a revenue share of 50.0% in 2023. Growth in the region can be attributed to the presence of technologically advanced contract research organizations and the increasing number of grants provided by the government organizations, such as the National Institute of Health (NIH), to foster research activities. The region’s CROs have established a good reputation and demonstrated exceptional performance, making them attractive for research investments during the forecast period. The U.S. market dominated the region in 2023. Extensive drug development activities, presence of several pharmaceutical & biotech companies, and surge in clinical trials in the country are some of the factors boosting the demand for in vivo research, thereby supporting the growth of the outsourced in vivo services. Furthermore, in June 2023, the U.S. administration granted USD 50 million to establish the Persistent Poverty Initiative to relieve the cumulative consequences of ongoing poverty on cancer development by building research capacity, promoting cancer prevention research, and promoting community-based programs.
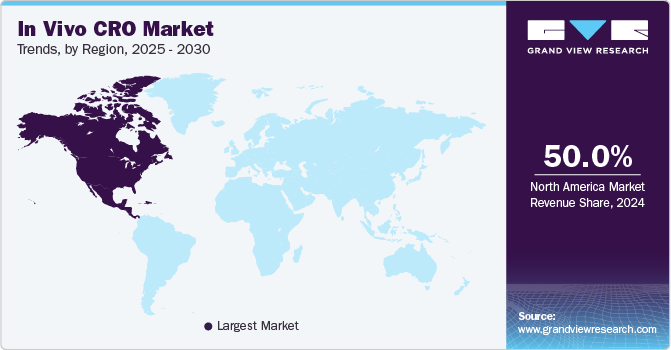
Asia Pacific is expected to witness the fastest CAGR during the forecast period. Growth in the region is due to several factors, including the cost-effectiveness of CROs across India and China, economic development, and advanced healthcare infrastructure. Increasing partnerships and investments by CROs and the growing percentage of outsourcing in vivo services to emerging economies are some factors expected to propel market growth during the forecast period. Moreover, in October 2023, Immunoadoptive Cell Therapy Private Limited announced the approval of the marketing authorization for its humanized CD19-targeted Chimeric Antigen Receptor T cell (CAR-T cell) therapy product for refractory/relapsed B-cell leukemia and lymphomas in India.
Key In Vivo CRO Company Insights
Some of the key players operating in the market include IQVIA Inc., Evotec, and Biocytogen Boston Corp, Crown Bioscience, and Charles River Laboratories. These companies are adopting organic and inorganic growth strategies, such as partnerships and collaborations and signing agreements for drug development and using R&D facilities to expand in the market.
SMO Clinical Research (I) Pvt Ltd and GemPharmatech are some of the emerging market participants in the market. These players are expanding their services and capabilities to meet the growing demand for in vivo CRO services. The companies are also involved in partnerships and product launches to strengthen their presence in the market.
Key In Vivo CRO Companies:
The following are the leading companies in the in-vivo CRO market. These companies collectively hold the largest market share and dictate industry trends. Financials, strategy maps & products of these in-vivo CRO companies are analyzed to map the supply network.
- IQVIA Inc.
- Crown Bioscience
- Taconic Biosciences, Inc.
- PsychoGenics Inc.
- Evotec
- Janvier Labs
- Biocytogen Boston Corp
- GemPharmatech
- Charles River Laboratories
- Icon Plc
- Labcorp Drug Development
- Parexel International Corporation
- SMO Clinical Research (I) Pvt Ltd.
- Comp14
- Comp15
Recent Developments
-
In November 2023, Charles River Laboratories announced the partnership with Aitia for drug development and in vivo oncology research.
-
In March 2023, Biocytogen Boston Corp announced a licensing agreement with Janssen Biotech, Inc. that granted Janssen Biotech, Inc. the rights to use the RenLite platform.
-
In January 2023, Evotec SE entered into a partnership agreement with Janssen Biotech to develop targeted immune-based therapies for oncology.
In Vivo CRO Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 4.98 billion
Revenue forecast in 2030
USD 7.93 billion
Growth rate
CAGR of 8.1% from 2024 to 2030
Actual Data
2018 - 2023
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million/billion, and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Model type, modality, indication, GLP type, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; South Korea; Australia; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
IQVIA Inc; Crown Bioscience; Taconic Biosciences, Inc.; PsychoGenics Inc.; Evotec; Janvier Labs; Biocytogen; GemPharmatech; Charles River Laboratories; Icon Plc; Labcorp Drug Development; Parexel International Corporation; SMO Clinical Research (I) Pvt Ltd.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global In Vivo CRO Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global in vivo CRO market report based on model type, modality, indication, GLP type, and region.
-
Model Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Rodent based
-
Rat Models
-
Mice Models
-
Others
-
-
Non-Rodent based
-
-
Modality Outlook (Revenue, USD Million, 2018 - 2030)
-
Small Molecules
-
Large Molecules
-
Cell & Gene Therapy
-
CAR T-cell therapies
-
CAR-NK cell therapy
-
TCR-T cell therapy
-
Other (Includes- TCR-NK, CARM, and TAC-T)
-
-
RNA Therapy
-
Others
-
-
-
Indication Outlook (Revenue, USD Million, 2018 - 2030)
-
Oncology
-
Blood cancer
-
Solid tumor
-
Syngeneic model
-
Patient derived xenograft
-
Xenograft
-
-
Others
-
-
CNS Conditions
-
Epilepsy
-
Parkinson's disease
-
Huntington's disease
-
Stroke
-
Muscular Dystrophy
-
Alzheimer’s Disease
-
Traumatic brain injury
-
Amyotrophic lateral sclerosis (ALS)
-
Spinal Muscular Atrophy
-
Muscle regeneration
-
Other Neurodevelopment Disorders
-
-
Diabetes
-
Obesity
-
Pain management
-
Chronic pain
-
Acute pain
-
-
Autoimmune/inflammation conditions
-
Rheumatoid Arthritis
-
Multiple Sclerosis
-
Osteoarthritis
-
Irritable Bowel Syndrome
-
Others
-
-
Others
-
-
GLP Type Outlook (Revenue, USD Million, 2018 - 2030)
-
GLP Toxicology
-
Non GLP
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. Some of the key market players include Pharmaceutical Product Development, LLC (PPD); Quintiles; ICON Plc; Parexel International; American Preclinical Services, LLC; Covance Inc.; Theorem Clinical research; WuXi AppTec, Inc.; inVentiv Health; Evotec (US), Inc.; and Charles River Laboratories.
b. Key factors that are driving the market growth include rising demand for advanced products, and changing regulatory landscape.
b. The global in vivo CRO market size was estimated at USD 4.59 billion in 2023 and is expected to reach USD 4.79 billion in 2023.
b. The global in vivo CRO market is expected to grow at a compound annual growth rate of 8.06% from 2024 to 2030 to reach USD 7.93 billion by 2030.
b. North America dominated the in vivo CRO market with a share of 50.0% in 2022. This is attributable to the availability of funding and grants from government organizations such as the National Institute of Health (NIH) to foster research activities.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





