- Home
- »
- Medical Devices
- »
-
Manual Resuscitators Market Size & Share Report, 2030GVR Report cover
![Manual Resuscitators Market Size, Share & Trends Report]()
Manual Resuscitators Market Size, Share & Trends Analysis Report By Type, By Modality, By Material, By Technology, By Patient Type, By Application, By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-1-68038-012-5
- Number of Pages: 100
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
The global manual resuscitators market size was valued at USD 514.0 million in 2022 and is expected to grow a compound annual growth rate (CAGR) of 5.7% from 2023 to 2030. The increasing prevalence of respiratory diseases across the globe and the growing demand for emergency medical devices are driving the market growth for manual resuscitators. A manual resuscitator, also known as a Bag Valve Mask (BVM) or self-inflating bag, is a device commonly deployed for positive ventilation in patients who are unable to breathe. Notable areas of application of manual resuscitators include ambulances, emergency rooms, and other critical care situations.
The market is expected to witness growth owing to the increasing incidence rates of cardiac arrest, increasing awareness initiatives, and the need for neonatal care. According to statistics from the World Health Organization published in June 2021, approximately 17.9 million individuals died from cardiovascular disorders in 2019, accounting for 32% of deaths globally. Of these, 75% of deaths were from low- and middle-income countries, and approximately 85% of deaths were owing to cardiac stroke and heart attack. These factors are likely to favor the growth of the market.
International organizations are coming together to reduce the chances of cardiac arrest cases and improve lives. The American Heart Association is collaborating with the World Health Organization to reduce premature deaths from non-communicable diseases. The goal of the initiative is to reduce the burden of non-communicable diseases by 25% by 2025, which will aid in decreasing the incidences of cardiovascular diseases and stroke globally. Such initiatives are anticipated to positively impact market growth.
Additionally, rising demand for neonatal care is expected to propel the growth of the market during the forecast period. According to the Centers for Disease Control and Prevention, preterm birth rates accounted for 10.1% in 2020 and 10.2% in 2019 in the U.S. Every 1 in 10 infants are born preterm in the country, and this increasing number of preterm births is poised to advance market growth.
According to the American Heart Association (AHA), around 10% of newly born infants require assistance for breathing at birth, among which less than 1% require intensive resuscitative steps to ensure the functionality of the heart and respiration. Hence, effective and necessary resuscitation during birth to these infants could aid in reduced rates of neonatal deaths and will boost the growth of the market further.
The COVID-19 pandemic had a positive impact on the market. Manual resuscitators were prioritized by the World Health Organization under the WHO medical device priority list in response to the COVID-19 pandemic.Although the pandemic affected the supply chain and logistics of manual resuscitators, a shortage of ventilators and a rise in the number of patients suffering from COVID surged its demand. Many countries utilized manual resuscitators to overcome the shortage of ventilators.
In the post-pandemic phase, the demand for manual resuscitators declined from this surge, owing to a reduction in the need for oxygen supply to patients. Technological advancements and increasing demand for low-cost, portable manual resuscitators to replace high-cost ventilators are poised to aid market growth during the forecast period. However, stringent government regulations pertaining to manual resuscitator use and manufacturing are expected to hinder the growth of the market. The market is highly regulated by the WHO, which provides technical specifications for manufacturing manual resuscitators.
Modality Insights
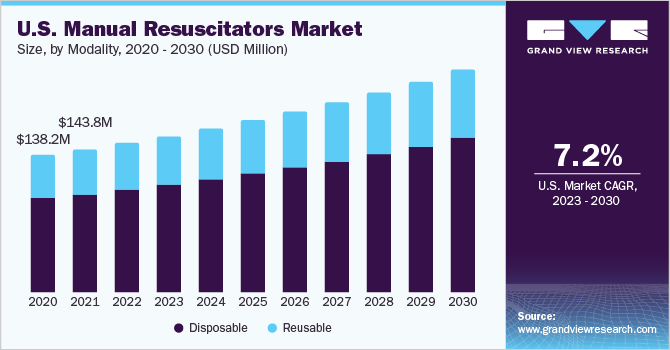
The disposable segment held the largest share in 2022. The segment is anticipated to witness considerable growth during the forecast period. Disposable resuscitators are witnessing high preference among patients and healthcare professionals as they have a low risk of infections. Rising cases of cardiopulmonary disorders are boosting the segment growth. Based on modality, the market is segmented into reusable and disposable.
Single-use or disposable resuscitation bags are usually made up of PVC while reusable ones are made of silicone. Reusable resuscitation bags can be cleaned, sterilized, and reassembled. However, they are usually more expensive than disposable ones but are more economical in the long term. Reusable silicone resuscitation bags are more environment-friendly, latex-free, and odorless. Furthermore, they have a better feel as they are softer. These advantages are expected to drive the growth of the reusable segment.
Material Insights
The silicone segment held the largest market share in 2022 due to its extensive use in the hospitals and outpatient setting for treating pediatric & adult patients suffering from heart & respiratory disease. Key manufacturers are extensively employing silicone-based manual resuscitators and innovations are majorly based on it. For instance, in September 2020, Aequs launched AQovent, a silicone-based, cost-effective, medical-grade, and mass-manufactured mechanical resuscitator. It is an emergency resuscitator driven by oxygen and enables constant pressurized ventilated flow automatically to the patients.
Based on material, the manual resuscitator market is classified as silicone, PVC, and rubber. The PVC material segment is projected to grow at the highest rate during the forecast period. Even though silicone resuscitators are more expensive when compared with PVC or rubber, in terms of long-term use, they are more economical when compared with disposable resuscitators. Owing to this, the demand for PVC is gradually increasing.
Technology Insights
Based on technology, the market is bifurcated into pop-off valves, PEEP valves, and others. Most of the resuscitators come only with the pressure valves and have a slot to accommodate the PEEP valve, and thus they fall into other categories. Generally, the resuscitators with just the pressure valve are used for treating medical conditions in adults. The PEEP valve gained attention during the COVID-19 pandemic due to higher oxygen level requirements in patients. Patients requiring oxygen of more than 0.5 FiO2 were suggested to use a PEEP valve while using manual resuscitators to improve oxygenation.
The other technologies segment held the largest market share in 2022 due to their extensive use in hospitals for treating adult patients. However, the pop-off valve segment is anticipated to grow at a significant rate over the forecast period. The demand for the pop-off valve is growing in NICUs as most of the neonates require respiratory support at birth.
Patient Type Insights
Based on patient type, the market is segmented into an adult, pediatric, and others. The adult segment held the largest share in 2022 due to the rising prevalence of Chronic Obstructive Pulmonary Disease (COPD) and cardiac diseases among this population, which includes a large geriatric patient pool. Another factor contributing to the growth of the adult segment could be the severe impact of the COVID-19 infection on adults and higher oxygen requirements for treatment. According to a study by Max Healthcare, oxygen requirements during the COVID-19 pandemic in India were around 63% in the first wave and 74% in the second wave.
According to data from the 2019 edition of World Population Prospects, there were around 703 million people aged 65 years and above in 2019 globally, and this number is expected to double to 1.5 billion by 2050. The other patient type segment includes neonates and infants. This segment is anticipated to witness remarkable growth during the forecast period, owing to the rising investments in neonatal intensive care to counter the growing incidences of neonatal deaths globally.
Application Insights
Based on application, the manual resuscitators market is segmented into Chronic Obstructive Pulmonary Disease (COPD), cardiopulmonary arrest, and others. The other segment includes the use of resuscitators during anesthesia and for treating conditions such as asthma. The COPD segment held the largest market share in 2022 owing to the rising burden of the disease. According to the WHO, in 2019, approximately 3.23 million people died due to COPD, making it the 3rd largest cause of death globally. Around 90% of these deaths occurred in low and middle-income countries.
On the other hand, the cardiopulmonary arrest segment is expected to gain momentum and expand at a rapid pace during the forecast period. This can be attributed to the growing awareness initiatives by governments and authorities about the use of manual resuscitators for cardiopulmonary treatment. For instance, the AHA has established the National Registry of Cardiopulmonary Resuscitation (NRCPR), which acts as a multi-site data collection source. The registry enables inter-facility comparison between various hospitals, establishing a standardized mechanism.
End-use Insights
Based on the end-use, the market is bifurcated into hospital, out-of-hospital, ASC, military, and others. The hospitals segment dominated the market in 2022. BVMs in hospitals are used in cases of neonatal care, cardiac arrest, and intensive care. The American Health Association (AHA) published the first guideline for reviewing, researching, and reporting in-hospital resuscitation. The in-hospital Utstein Style was published as a mechanism of international standardization and is used as a reporting system for patient monitoring.
The out-of-hospital segment is expected to witness lucrative growth owing to the increasing need to provide bystander cardiopulmonary resuscitation and rising awareness initiatives. The low survival rate of out-of-hospital CPR is driving the need for manual resuscitators. The rising adoption of ambulatory surgical centers is anticipated to boost the segment growth. Ambulatory surgical centers provide instant surgical care and can save valuable time for patients. The increasing number of ASCs and rising cases of cardiac arrest are propelling segment growth.
Type Insights
The self-inflating segment dominated the market with a share of around 47.9% in 2022, owing to their usability in hospital emergency departments such as delivery wards & neonatal units and the increasing launches of self-inflating resuscitators. For instance, in April 2020, the Vecna family of companies, including Vecna Healthcare, Vecna Robotics, and VecnaCares, collaborated with MIT CSAIL and 10XBeta, among others, to launch the automated manual resuscitator Ventiv. Ventiv automates the compression and relaxation of manual bags to offer constant pressure ventilation and can be used with Ambu bags or any other self-inflating bag.
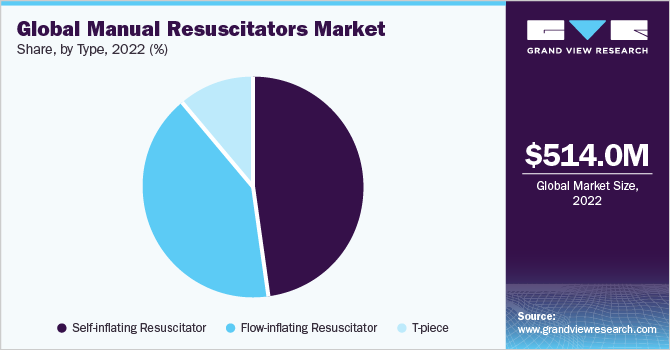
The flow-inflating bags segment is expected to advance with the fastest CAGR of 6.3% over the forecast period. Flow-inflating bags require an oxygen reservoir to ensure 100% oxygen flow. Flow-inflating resuscitators are majorly used in neonatal care and intensive units. According to a World Health Organization report in 2022, approximately 47% of deaths of children under 5 years of age occur in the neonatal stage. The major cause of death is birth asphyxia, inability to breathe at birth, or some birth defects. Thus, the rising prevalence of asphyxia in neonates is expected to boost segment growth.
The T-piece segment is expected to witness lucrative growth during the forecast period owing to its benefits compared to flow-inflating bags. According to a study of pediatric research, in 2020, when compared to Self-Inflating Bags (SIBs), T-piece resuscitators (TPR) provided more consistent peak inspiratory pressure. On the other hand, SIBs usually produce less positive end-expiratory pressure (PEEP) than the required and set standards, and delivery varies depending on the set PEEP, respiratory rate, gas flow, and SIB + PEEP valve model.
Regional Insights
North America held the maximum market share of 37.6% in 2022. This can be attributed to the increasing cases of cardiac arrest and requirement of neonatal ventilation in the region. For instance, in November 2020, the first virtual Resuscitation Science Symposium was organized in the U.S. with the aid of the American Heart Association. The symposium was organized to discuss the latest developments in resuscitation research and to share knowledge among peers.
Moreover, initiatives to control out-of-hospital cardiac arrests are expected to drive the regional market growth. For instance, research networks, such as the Resuscitation Outcomes Consortium (ROC), have been established to study better ways to treat outside hospital cardiac arrest and trauma cases. The ROC is a clinical research network of ten regional centers throughout the U.S. and Canada.
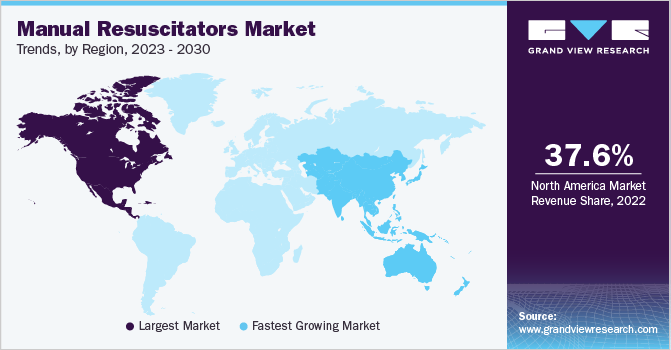
Asia Pacific is expected to expand at the fastest CAGR of 7.0% during the forecast period. The Government of India has undertaken initiatives to improve in-hospital neonatal care under the National Health Mission 2015 and New-born Action Plan (NAP). Under this initiative, the government of India is expected to provide 2,706 hospitals as first referral units, 14,000 newborn care corners, and 2,020 newborn stabilization units. Under NAP, the Government of India aims to reduce neonatal deaths and bring the rate down to single-digits by 2030. Such initiatives are anticipated to fuel market growth in the region.
Key Companies & Market Share Insights
The market is consolidated in nature with few prominent players acquiring the major portion of the market. Product launches, investments in R&D, and the expansion of product portfolios are strategies undertaken by key players to strengthen their market position. For instance, in September 2020, Aequs launched AQovent, a silicone mechanical resuscitator, developed in collaboration with the University of Illinois. It is a medical grade, mass produced, and low-cost mechanical resuscitator.
Similarly, in July 2018, Medline industries acquired NeuroGym Technologies, a therapy and rehabilitation equipment manufacturer based in Ottawa. With this acquisition, Medline aims to focus on offering improved solutions for post-acute healthcare in North America. Some of the prominent industry players operating in the global manual resuscitators market include:
-
WEINMANN Emergency Medical Technology GmbH + Co. KG
-
Laerdal Medical
-
Ambu A/S
-
Medline Industries, LP
-
Hopkins Medical Products
-
ResMed, Inc.
-
HUM Gesellschaft für Homecare und Medizintechnik mbH
-
PERSYS MEDICAL
-
CareFusion
Manual Resuscitators Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 540.4 million
Revenue forecast in 2030
USD 799.3 million
Growth Rate
CAGR of 5.7% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
September 2023
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, modality, material, technology, patient type, application, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Key companies profiled
WEINMANN Emergency Medical Technology GmbH + Co. KG; Laerdal Medical; Ambu A/S; Medline Industries, LP; Hopkins Medical Products; ResMed, Inc.; HUM Gesellschaft für Homecare und Medizintechnik mbH; PERSYS MEDICAL; CareFusion
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Manual Resuscitators Market Report Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global manual resuscitators market report based on type, modality, material, technology, patient type, application, end-use, and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Self-inflating Resuscitator
-
Flow-inflating Resuscitator
-
T-piece
-
-
Modality Outlook (Revenue, USD Million, 2018 - 2030)
-
Disposable
-
Reusable
-
-
Material Outlook (Revenue, USD Million, 2018 - 2030)
-
Silicon
-
PVC
-
Rubber
-
-
Technology Outlook (Revenue, USD Million, 2018 - 2030)
-
Pop-off valve
-
PEEP valve
-
Others
-
-
Patient Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Adult
-
Pediatric
-
Others
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Chronic Obstructive Pulmonary Disease
-
Cardiopulmonary Arrest
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospital
-
Out-of-hospital
-
ASC
-
Military
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Norway
-
Denmark
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global manual resuscitators market size was estimated at USD 514.0 million in 2022 and is expected to reach USD 540.4 million in 2023.
b. The global manual resuscitators market is expected to grow at a compound annual growth rate of 5.75% from 2023 to 2030 to reach USD 799.3 million by 2030.
b. North America dominated the manual resuscitators market with a share of 37.6% in 2022. The introduction of state-of-the-art resuscitation products and training kits to reduce the chances of delayed ventilation are the major drivers of the market.
b. Some key players operating in the manual resuscitators market include WEINMANN Emergency Medical Technology GmbH + Co. KG; Laerdal Medical; Ambu A/S; Medline Industries, LP; Hopkins Medical Product; ResMed, Inc.; HUM Gesellschaft für Homecare und Medizintechnik mbH; PERSYS MEDICAL, and CareFusion
b. Key factors that are driving the manual resuscitators market growth include increasing incidence rates of cardiac arrest, increasing awareness initiatives and simulation websites, and the need for neonatal care.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





