- Home
- »
- Medical Devices
- »
-
Medical Device Analytical Testing Outsourcing Market, 2030GVR Report cover
![Medical Device Analytical Testing Outsourcing Market Size, Share & Trends Report]()
Medical Device Analytical Testing Outsourcing Market Size, Share & Trends Analysis Report By Service (Sterility Testing, Physical Testing), By Therapeutic Areas, By Device Type, By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-1-68038-366-9
- Number of Pages: 133
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
The global medical device analytical testing outsourcing market size was valued at USD 5.66 billion in 2022 and is anticipated to grow at a compound annual growth rate (CAGR) of 8.2% from 2023 to 2030. Key factors driving the market growth include complexity in product design, increasing competition, the number of small-scale medical device manufacturers, and strict approval norms. The COVID-19 pandemic has emphasized the significance of developing novel low-cost medical equipment. This crisis has resulted in a global economic and health pandemic. As a result, several enterprises have shut down their manufacturing facilities and halted the majority of the manufacturing process. Pricing pressure in the medical device industry is expected to create a growth opportunity for the market.
In developed countries, such as the U.S., there is pricing pressure, hence, operators are exploring every possible way to reduce costs throughout the value chain. On the other hand, it is in developing economies where the actual potential lies. However, developing regions are likely to be price-sensitive. Hence, market players are striving hard to reduce the overall cost of devices. Outsourcing analytical testing operations helps companies focus on product development and enhancing marketing efforts. Furthermore, an increasing number of small-scale medical device manufacturers lacking in-house testing capabilities is expected to boost market growth.
Challenges associated with small- and medium-sized entities include the lack of in-house infrastructure, knowledge, and expertise required to successfully integrate IT components into products. As a result, companies are outsourcing a significant portion of their manufacturing operations to established CMOs. CMOs offer substantial cost savings, access to sophisticated infrastructure, large production capacities, and rapid time-to-market.The regulatory authorities also conduct routine post-market surveillance by charging the manufacturers a fee. The receipt of any complaints regarding the product’s flaws results in its removal from the market, demonstrating the stringency of these procedures.
Personalized medicine, drug-device combinations, Artificial Intelligence (AI), wearables, and a greater emphasis on real-time patient monitoring have all resulted in a complicated medical device ecosystem. When it comes to chronic disease management, such as diabetes and Cardiovascular Diseases (CVDs), real-time patient monitoring, remote patient monitoring, and continuous patient monitoring are the primary areas of focus.The medical device industry is witnessing fierce competition, with companies using a mix of offensive and defensive marketing strategies to maintain their position in the short as well as long term.
Some of these strategies include the launch of new products, extensive R&D, competitive pricing, collaborative development, geographic expansion, and mergers & acquisitions. For instance, in June 2022, Mayo Clinic Platform entered into a partnership with Becton, Dickinson, and Company (BD), a medical technology company to conduct an in-depth post-market analysis of BD's medical device offerings. This initiative aims to foster healthcare innovation. This initiative will use Mayo Clinic Platform_Discover, one of the platform's de-identified patient datasets.
Service Insights
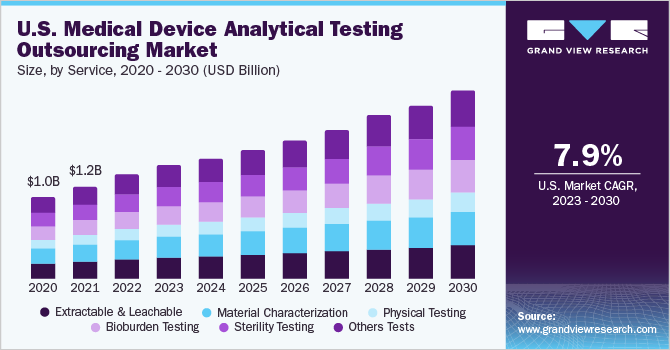
The material characterization segment dominated the market and accounted for the largest revenue share of 18.2% in 2022. Material characterization forms the basis for understanding the composition of a medical device material and its potential to cause an undesirable biological effect when used. It also acts as a technique for ensuring material uniformity from one lot of devices to the next. As the ISO 10993 standards and FDA criteria become more aligned, the approaches outlined above would be employed to a larger extent by the U.S. device industry to aid in the selection of ideal materials and to control the uniformity of medical devices.
The other testing segment is expected to rise with the fastest CAGR over the forecast period. Other tests include cleaning, reprocessing, method development & validation, accelerated stability testing, biocompatibility, validation testing for packaging materials (container closure permeation), and transport stability.Usually, a comprehensive package is offered by vendors to their clients to cater to all the needs of a single entity. Hence, contractual studies are expected to grow over the forecast period, thereby boosting the others test segment growth.
Device Type Insights
The other segment accounted for the largest revenue share of 79.55% in 2022. The other segment includes single-use, expensive therapeutic and monitoring devices. High shares of the segment can majorly be attributed to the increasing demand for contract outsourcing services across the globe. Moreover, the increasing number of medical devices being developed for niche specialties that require efficient testing is one of the major factors supporting segment growth.
On the basis of device types, the global industry has been further categorized into reprocessed devices and other devices. The reprocessed devices segment is estimated to register the fastest growth rate of 9.8% during the forecast period. Reprocessing aims to save costs, which often jeopardizes patient safety. In the developed regions, particularly in the U.S. and Europe, it is a systematic science authorized by the U.S. FDA that offers to reduce long-term healthcare costs and provide cost savings.
Therapeutic Areas Insights
The cardiology segment accounted for the maximum revenue share of more than 21.00% in 2022. Rising demand for cardiovascular devices, as a result of the increasing prevalence of related conditions, is attributable to the expansion of these products outsourcing. As per the Centers for Disease Control and Prevention statistics in 2021, 695,000 people died from heart disease in the U.S. Furthermore, the significant complexity of cardiovascular devices, as well as the necessity for technical skills, leads to increased outsourcing of these devices.
On the other hand, the general & plastic surgery segment is expected to rise with the fastest CAGR over the forecast period. Growing demand for cosmetic surgeries is expected to boost the outsourcing of general as well as cosmetic surgical devices. According to data from the Aesthetic Society, American consumers spent more than USD 6 billion on cosmetic surgery in 2020 and more than USD 3 billion on nonsurgical aesthetic services. Also, during the pandemic-induced lockdown, cosmetic doctors and plastic surgeons around the world reported an increase in bookings for surgical and non-surgical treatments.
End-use Insights
The hospitals segment dominated the global market in 2022 and accounted for the maximum share of more than 87% of the global revenue. This growth can be attributed to the increased patient traffic and increased budget allocation for these operations by hospital administration. Increasing demand for novel technology-based medical devices across hospital facilities is another significant factor supporting the adoption of analytical testing for such devices. The facilities incorporate innovative new technology-based medical devices, which require efficient analytical testing for enhanced performance, thus augmenting the market growth. Moreover, hospital facilities are the major sources of refurbished medical devices that require analytical services, which makes them one of the most significant platforms that outsource these services to contract developers.
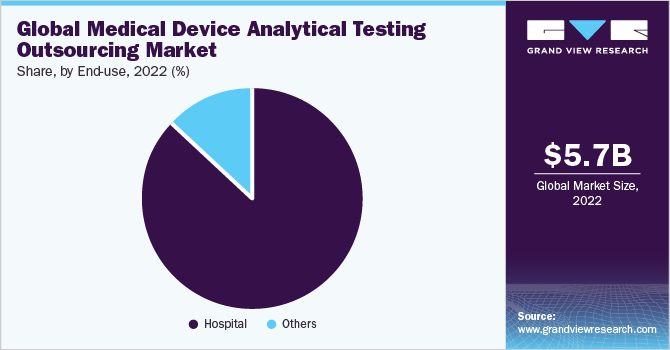
Other healthcare settings, such as diagnostic centers, ambulatory care centers, and specialty care centers, are also expected to gain significant market shares in the years to come. The hospitals segment is further categorized into equipment and consumables. In 2022, the equipment category accounted for approximately 39.20% of hospital-based medical equipment analytical testing services. Cardiology equipment, imaging equipment, surgical microscopes, surgical devices, and therapeutic & monitoring devices are examples of these devices. These devices are acquired for long-term usage and have calibration, preventive maintenance, and performance checks outsourced.
Regional Insights
Asia Pacific dominated the global market in 2022 and accounted for more than 40.69% of the global revenue share. The regional market is estimated to expand further at the fastest CAGR retaining its leading position throughout the forecast period. This growth can be attributed to government efforts to improve healthcare infrastructure in the region. Furthermore, economic changes in India and China are likely to support market expansion. Due to the region’s large population base and low per capita income, there is a great demand for economical treatment solutions. Multinational corporations are eager to invest in developing countries, such as India and China.
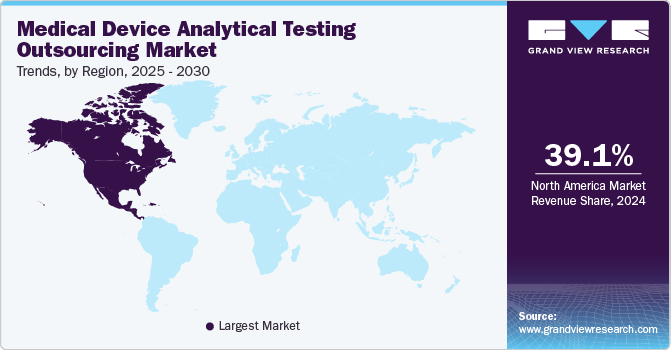
North America is also anticipated to witness considerable growth during the forecast period. The growth of this regional market is mainly credited to the fact that it is one of the most important production centers for extremely reliable, sophisticated, and high-end medical products. As a result, Original Equipment Manufacturers (OEMs) are increasingly turning to electronics manufacturing service providers to handle the growing volume of electronic components in today’s medical devices. A rapid increase in the production of medical devices to fulfill the region’s growing need for effective healthcare is projected to be one of the primary factors driving the market growth.
Key Companies & Market Share Insights
Mergers, acquisitions, and partnerships among others are the key strategies adopted by industry participants to maintain their market share. For example, in December 2021, Toxikon Corp. was acquired by LabCorp. The company’s strategic location enables LabCorp to expand its nonclinical work with large pharmaceutical companies and biotech firms in the region, as well as facilitate entry into medical device Investigational Device Exemption (IDE) filings. Moreover, in November 2022, to create an integrated full-function service group, Medeologix successfully acquired three leading medical device manufacturing companies. With a total area of 90,000 square feet, the newly established Medeologix group of firms provides one-stop services for everything from high-volume production to conceptual design. Some of the prominent players in the global medical device analytical testing outsourcing market include:
-
SGS
-
Toxikon, Inc.
-
Eurofins Scientific
-
Pace Analytical Services LLC
-
Intertek Group plc
-
Wuxi AppTec
-
North American Science Associates, Inc.
-
Envigo
-
Charles River Laboratories International, Inc.
-
Medical Device Testing Services
Medical Device Analytical Testing Outsourcing Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 6.09 billion
Revenue forecast in 2030
USD 10.57 billion
Growth rate
CAGR of 8.2% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Service, device type,therapeutic areas, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
SGS; Toxikon, Inc.; Eurofins Scientific; Pace Analytical Services LLC; Intertek Group plc; Wuxi AppTec; North American Science Associates, Inc.; Envigo; Charles River Laboratories International, Inc.; Medical Device Testing Services
Customization scope
Freereport customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, and segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Medical Device Analytical Testing Outsourcing Market Report Segmentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the medical device analytical testing outsourcing market based on service, device type, therapeutic areas, end-use, and region:
-
Service Outlook (Revenue, USD Billion, 2018 - 2030)
-
Extractable & Leachable
-
Material Characterization
-
Physical Testing
-
Bioburden Testing
-
Sterility Testing
-
Other Tests
-
-
Device Type Outlook (Revenue, USD Billion, 2018 - 2030)
-
Reprocessed Devices
-
Others
-
-
End-use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Hospital
-
Others
-
-
Therapeutic Areas Outlook (Revenue, USD Billion, 2018 - 2030)
-
Cardiology
-
Diagnostic Imaging
-
Orthopedic
-
IVD
-
Ophthalmic
-
General & Plastic Surgery
-
Drug Delivery
-
Endoscopy
-
Dental
-
Diabetes Care
-
Others
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
India
-
China
-
Japan
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global medical device analytical testing outsourcing market size was estimated at USD 5.66 billion in 2022 and is expected to reach USD 6.09 billion in 2022.
b. The global medical device analytical testing outsourcing market is expected to grow at a compound annual growth rate of 8.2% from 2023 to 2030 to reach USD 10.57 billion by 2030.
b. The Asia Pacific dominated the medical device analytical testing outsourcing market with a share of 41.7% in 2022 This is attributable to the fact that it is one of the top manufacturing hubs of highly reliable, complex, and high-end medical devices.
b. Some key players operating in the medical device analytical testing outsourcing market include SGS SA; Toxikon, Inc.; Eurofins Scientific; Pace Analytical Services, LLC; Intertek Group plc; WuXi AppTec.; NORTH AMERICAN SCIENCE ASSOCIATES INC.; Envigo; Charles River Laboratories International Inc.; and Medical Device Testing Services.
b. Key factors that are driving the medical device analytical testing outsourcing market growth include complexity in product design, intensifying competition, an increasing number of small-sized medical device manufacturers lacking in-house testing infrastructure.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





