- Home
- »
- Medical Devices
- »
-
Middle East Medical Device Market Size, Industry Report, 2018-2025GVR Report cover
![Middle East Medical Device Market Size, Share & Trends Report]()
Middle East Medical Device Market Size, Share & Trends Analysis Report By Product (In Vitro Diagnostics (IVD), Cardiology, Orthopedics), By FDA classification (Class I, II, III), By End-user, By Region, And Segment Forecasts, 2018 - 2025
- Report ID: GVR-1-68038-548-9
- Number of Pages: 165
- Format: Electronic (PDF)
- Historical Range: 2014 - 2016
- Industry: Healthcare
Industry Insights
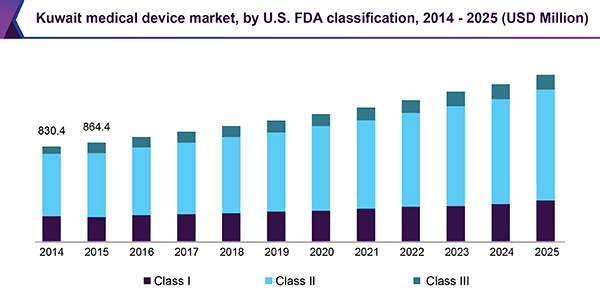
The Middle East medical device market size was valued at USD 19.6 billion in 2016 and is expected to grow at a CAGR of over 5.5% over the forecast period. This growth is anticipated to be due to dynamic innovations and technological advancements. The majority of the Middle Eastern countries are early adopters of technological advancements and thus provide extensive opportunities for medical device firms. Technological breakthroughs concentrating on increasing efficiency and accuracy are expected to occur during the forecast period.
Supportive reimbursement pathways are vital to impact rendering driver for medical devices in the Middle East. These pathways are established to provide quality healthcare facilities to the civilians of Middle Eastern countries. Favorable pathways such as the National Health Insurance program and private healthcare reimbursement systems cater to the basic healthcare needs of Middle Eastern citizens.
Rising incidences of conventional diseases such as diabetes and obesity is another driver for the growth of medical devices in Middle Eastern countries. The World Health Organization (WHO), estimated a total of 422.0 million people suffering from diabetes across the globe out of which 43 million are estimated to be from the Middle Eastern region.
Product Insights
The Middle East medical device market has been analyzed based on In-Vitro Diagnostics(IVD), Cardiology, Orthopedics, Diagnostic imaging, Ophthalmic, General and plastic surgery, Drug delivery, Endoscopy, Dental, Wound management, Diabetic care, Nephrology, Ear, Nose and Throat, General hospital and Healthcare, Neurology, Skin Care & Aesthetics and other products. In-Vitro Diagnostics (IVD) dominated the segment as of 2016, with over 18.0% of the market share.
The factors responsible for the dominance of this segment in the field of medical devices in the Middle East include an increased rate of a sedentary lifestyle, thus, increasing the rate of conventional diseases such as diabetes, cardiovascular diseases, obesity, and many more. Also, the rising rate of the geriatric population results in higher demand for IVDs in the Middle East.
The neurology devices segment is anticipated to witness maximum growth during the forecast period with a CAGR of over 14.2%. This extensive growth is predicted due to increasing investments in R&D of neurological devices and technologies. The increasing availability of sophisticated equipment to monitor and track brain and nerve activity to facilitate better diagnostics and treatments to patients is a major driver for the growth of this segment in the Middle East.
U.S. FDA Classification Insights
Based on the U.S. FDA classification, the Middle East medical devices market is segmented into Class I, II, and III devices. As of 2016, Class II forms the largest as well as the fastest-growing segment over the forecast period.
Over 60.0% of medical devices fall under the Class II category. Formerly, Class I devices were predominant with an extensive amount of market players. This has led to saturation of the market and a standstill in the growth, as per innovation is concerned.
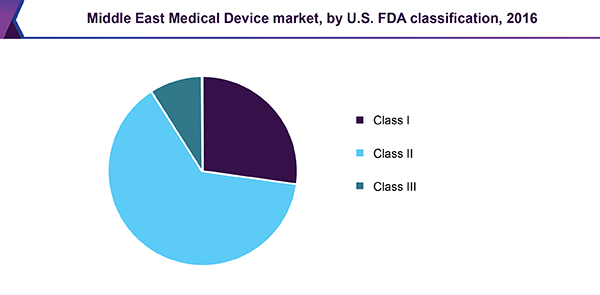
Class II devices are higher risk devices that require greater regulatory controls and are more critical as compared to Class I devices. Thus, self-declaration is illegal. An increased growth rate is witnessed in this segment due to rising incidences of conventional diseases in the Middle Eastern region. This results in an increased demand for technological advancements for more efficacious and high precision devices.
End-user Insights
Based on end-users, the Middle East medical devices market is segmented into hospitals, clinics, laboratories, blood banks, pharmacies, standalone clinics, dermatology clinics & cosmetic centers.
As of 2016, hospitals are the predominant segment with over 60.0% of the market share. This is anticipated to be due to the increasing patient footfall in a hospital as compared to any other end-user segment. The number of hospitals is increasing in the Middle Eastern region due to the increasing population and increasing demand for diagnosis and treatment. In the 2016 budget, the Middle East government allocated USD 27.7 billion towards further development of the hospital sector.
The dermatology clinics & cosmetic center segments are anticipated to witness maximum growth during the forecast period with a CAGR of over 6.0%. Rising demand for non-invasive dermatological and cosmeceutical procedures along with increasing aesthetic awareness has accelerated the adoption of dermatology and cosmetology centers in the Middle East.
Regional Insights
Regionally, the Kingdom of Saudi Arabia is the most dominant one and is the largest revenue generator for the Middle East medical device market, as of 2016. This dominance is anticipated to be due to enhanced public sector spending, with more than 60.0% share contributed by the Ministry of Health (MoH). An increase is witnessed in budget allocation and expansion in the facilities such as hospitals and clinics catering to the increasing population.
On the other hand, Qatar is anticipated to witness the fastest growth over the forecast period.
Factors identified to support this growth include the rapidly evolving healthcare infrastructure and inclusion of research activities by the medium as well as small scale players. Increasing urbanization, expanding the health insurance market, growing population, and tremendous healthcare developments are the major drivers resulting in the growth of medical devices in this region. Furthermore, the augmenting figure of patients within the region coupled with flexible regulatory guidelines are expected to promote the region as a key destination.
Middle East Medical Device Market Share Insights
A few of the key market participants in the Middle East medical devices market include Medtronic, Johnson & Johnson, Siemens, Roche, Becton Dickinson, Abbott Laboratories, Novartis, Olympus Corporation, General Electric, Biomerica, Baxter International, Smith and Nephew, BioMérieux, Philips and Zimmer Biomet.
Owing to the augmenting rate of outsourcing activities within the healthcare industry, entry of various new players coupled with collaborative efforts by existing players is expected to be witnessed over the forecast period.
For instance, In December 2016, Becton Dickinson announced a research collaboration with a leading global organization “Juvenile Diabetes Research Foundation (JDRF)” that would be funding Becton Dickinson’s research for type 1 diabetes. This is anticipated to enable BD’s focus on enhancing technology supporting extended wear insulin infusion set.
In September 2016, Abbott agreed to sell Abbott Medical Optics to Johnson & Johnson for USD 4.3 billion in cash. Other strategic plans are the development of technologies and innovative devices to serve the current unmet needs.
Report Scope
Attribute
Details
The base year for estimation
2016
Actual estimates/Historical data
2014 - 2016
Forecast period
2017 - 2025
Market representation
Revenue in USD Million & CAGR from 2017 to 2025
Regional scope
Middle East
Country scope
Saudi Arabia, U.A.E., Qatar, Kuwait, Egypt, Bahrain, Cyprus, Iran, Iraq, Oman.
Report coverage
Revenue forecast, company share, competitive landscape, growth factors, and trends
15% free customization scope (equivalent to 5 analysts working days)
If you need specific market information, which is not currently within the scope of the report, we will provide it to you as a part of customization
Segments Covered in the ReportThis report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2014 to 2025. For this study, Grand View Research has segmented the Middle East medical device market based on the type of device, U.S. FDA classes, end-user, and region:
-
Type of devices (Revenue, USD Million, 2014 - 2025)
-
In Vitro Diagnostics(IVD)
-
Cardiology
-
Orthopedics
-
Diagnostic imaging
-
Ophthalmic
-
General and plastic surgery
-
Drug delivery
-
Endoscopy
-
Dental
-
Wound management
-
Diabetic care
-
Nephrology
-
Ear, Nose, and Throat
-
General hospital and Healthcare
-
Neurology
-
Skin Care & Aesthetics
-
Others
-
-
U.S. FDA classification (Revenue, USD Million, 2014 - 2025)
-
Class I
-
Class II
-
Class III
-
-
The end-user (Revenue, USD Million, 2014 - 2025)
-
Hospitals
-
Clinics
-
Laboratories
-
Blood Banks
-
Pharmacies
-
Stand Alone Clinics
-
Dermatology Clinics & Cosmetic
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2014 - 2025)
-
Saudi Arabia
-
UAE
-
Qatar
-
Kuwait
-
Oman
-
Bahrain
-
Egypt
-
Iran
-
Iraq
-
Israel
-
Cyprus
-
Jordan
-
Turkey
-
Lebanon
-
Palestine
-
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





