- Home
- »
- Medical Devices
- »
-
Structural Heart Devices Market Size & Share Report, 2030GVR Report cover
![Structural Heart Devices Market Size, Share & Trends Report]()
Structural Heart Devices Market Size, Share & Trends Analysis Report By Type (Surgical Aortic Valve Replacement, Transcatheter Aortic Valve Replacement, Mitral Repair, Left Atrial Appendage Closure), By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-1-68038-785-8
- Number of Pages: 90
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
The global structural heart devices market size was valued at USD 6.33 billion in 2022 and is anticipated to grow at a compound annual growth rate (CAGR) of 9.3% from 2023 to 2030. The high prevalence of target diseases is the key driver of the market. Almost 60 million people in the U.S. have structural defects in their hearts. This accounts for around 20-25% of the country’s adult population. This indicates the huge scope of these devices in addressing structural heart defects. Structural heart disease (SHD) treatment includes a wide category of percutaneous treatments for patients with both acquired heart disease and congenital heart disease (CHD) that pertain to functional and structural abnormalities of cardiac chambers, proximal great vessels, and heart valves.
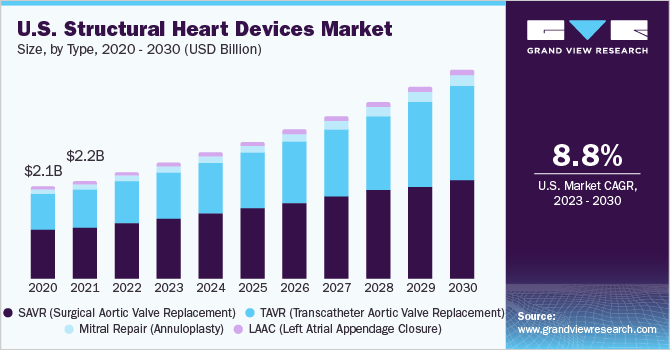
The reimbursement scenario in the U.S. is likely to become more favorable in the coming years, thus making procedures such as TAVR (Transcatheter Aortic Valve Replacement) affordable. As the target population ages, their medical expenditure is covered under the Centers for Medicare and Medicaid Services (CMS). It eases the burden of healthcare expenditure, thereby encouraging more people to opt for these treatments. This is a positive impact-rendering factor for procedures such as Left Atrial Appendage Closure (LAAC), which are useful for the reduction of the risk of stroke.
Industry players are investing heavily in research and development procedures to launch effective products and remain competitive in the market. Medtronic, Boston Scientific, Abbott, and Edwards Lifesciences, for instance, represented a few of the organizations and revealed new data for their cardiovascular offerings at the 34th Transcatheter Cardiovascular Therapeutics (TCT) conference held in Boston in September 2022.
The market is likely to be driven by the surge in the number of people suffering from structural heart diseases. As these diseases are usually congenital, they frequently occur among infants. Tissue-enhanced aortic valves are helpful in structural replacement and repair of the heart and hence people with these diseases are moving toward such innovations. The advent of technology has enhanced the functioning of structural heart devices. The improved treatments have raised the standard of living and increased the life expectancy of people. Since structural heart surgeries are minimally invasive, they are chosen over traditional invasive procedures such as open-heart surgeries.
According to the Children's HeartLink organization, approximately 1 out of every 100 infants has congenital cardiac disorders, which can vary from moderate to severe. As per Cleveland Clinic, mitral valve regurgitation is the most commonly occurring valve disorder in the U.S. Such facts demonstrate the necessity of structural heart diagnosis devices, which creates extensive opportunities for industry players.
However, the adoption of advanced structural heart devices has been sluggish, particularly in developing countries, as these devices are costlier than their traditional equivalents. Stroke, renal failure, and gastrointestinal issues are all complications connected with these devices, which occur following a mitral valve replacement treatment. The high cost of modern structural heart implants, as well as the risks associated with these treatments, are major market restraints.
The COVID-19 pandemic disrupted routine care in many medical facilities and had an impact on healthcare systems around the world. This significantly increased the risks faced by susceptible patients with cardiovascular disorders. On the other hand, due to the increased risk of infection among cardiovascular patients, the demand for various cardiovascular devices, particularly structural cardiac devices, rose during the pandemic.
Type Insights
The SAVR segment dominated the market with a revenue share of 51.8% in 2022 due to its proven efficacy in treatment procedures and well-established reimbursement codes. SAVR also has clearly outlined surgical guidelines, which makes it a preferred choice for surgeons as they can refer to it during procedures. Based on the type of device, the market is segmented into surgical aortic valve replacement (SAVR), transcatheter aortic valve replacement (TAVR), mitral repair, and left atrial appendage closure (LAAC).
The TAVR segment, on the other hand, is anticipated to expand at the fastest CAGR of 10.2% over the forecast period, as it is a minimally invasive procedure that can be used to replace a narrowed aortic valve without the need for open-heart surgery. As the prevalence of aortic stenosis (AS) increases, the demand for TAVR procedures is also likely to rise. In addition, TAVR is less invasive than traditional open-heart surgery. This makes it a more attractive option for patients who are at high risk for complications from open-heart procedures.
Newer TAVR devices are smaller, more flexible, and easier to implant than older devices. This has made TAVR a more viable option for a wider range of patients. In May 2023, a study published in the Journal of the American College of Cardiology claimed that minimally invasive TAVR procedures produced consistently better clinical results than open heart surgery over a 3-year follow-up period. The study was conducted to assess the long-term outcomes in aortic stenosis patients who underwent TAVR as a part of the Evolut Low Risk trial.
Regional Insights
North America dominated the global structural heart devices market and held the largest revenue share of 40.8% in 2022, owing to an increase in healthcare spending, increasing research and development activities, and a rising prevalence of heart diseases due to a steady growth in the geriatric population in the region. A strong preference for minimally invasive procedures is also the key reason for the regional dominance.
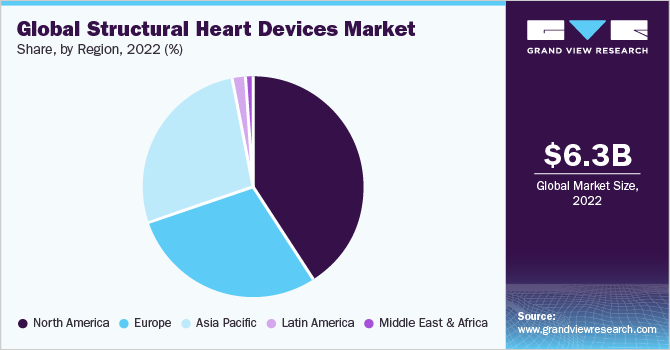
According to data published by Cedars-Sinai in January 2022, the most prevalent type of heart surgery is coronary artery bypass graft surgery (CABG)/coronary artery bypass/bypass surgery. Every year, more than 300,000 patients in the U.S. successfully undergo bypass surgery. This demonstrates the growing prevalence of cardiovascular disorders in the nation.
On the other hand, the Asia Pacific region is anticipated to advance at the fastest CAGR of 10.0% during the forecast period. This can be attributed to the rise in healthcare expenditure, increasing awareness about cardiac diseases, better newborn screening programs, and improvement in healthcare access in the region. In addition, due to improved life expectancy and low pricing of these products, the regional demand for structural heart devices is expected to increase in the coming years.
Manufacturers of these devices are looking into the untapped development potential in this region. For instance, in December 2022, Abbott announced the launch of Navitor in India. It is a minimally-invasive transcatheter aortic valve implantation (TAVI) system, indicated for people with severe aortic stenosis who are at high or extreme surgical risk. The innovative device prevents blood from leaking around the valve, advancing transcatheter aortic valve replacement therapies.
Key Companies & Market Share Insights
The industry is witnessing steady growth as new players are entering the market. While there are new entrants, the existing players are strengthening their foothold by launching novel products and entering into mergers & acquisitions. For instance, in March 2023, Abbott announced the U.S. FDA approval for its stented tissue valve, Epic Max, to treat patients with stenosis or aortic regurgitation. This product is the newest addition to Abbott's Epic surgical valve platform with a proven history of positive clinical results and reliability. Its optimized design is claimed to further enhance valve circulation.
In another development, in September 2022, Edwards Lifesciences announced the FDA approval of its advanced SAPIEN 3 Ultra RESILIA TAVR valve. The device integrates the company’s RESILIA tissue technology with the SAPIEN 3 Ultra TAVR device. This upgraded tissue was created to lower the risk of reintervention in TAVR patients and is anticipated to be a crucial component of the next generation of Edwards valves. In May 2022,Biomerics, a leading U.S.-based medical device contract manufacturer, announced its expansion in Ireland with the inauguration of the Balloons & Balloon Catheters Centre of Excellence in the western city of Galway. Some of the prominent players in the global structural heart devices market include:
-
Boston Scientific Corporation
-
Medtronic
-
Edwards Lifesciences Corporation
-
Abbott Laboratories
-
ST. JUDE MEDICAL
-
Biomerics
-
Comed BV
-
LivaNova PLC
-
JenaValve Technology, Inc.
-
CardioKinetix
Structural Heart Devices Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 6.9 billion
Revenue forecast in 2030
USD 12.8 billion
Growth rate
CAGR of 9.3% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
September 2023
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Key companies profiled
Boston Scientific Corporation; Medtronic; Edwards Lifesciences Corporation; Abbott Laboratories; ST. JUDE MEDICAL; Biomerics; Comed BV; LivaNova PLC; JenaValve Technology, Inc.; CardioKinetix
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Structural Heart Devices Market Report Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global structural heart devicesmarket report based on type and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
SAVR (Surgical Aortic Valve Replacement)
-
TAVR (Transcatheter Aortic Valve Replacement)
-
Mitral Repair (Annuloplasty)
-
LAAC (Left Atrial Appendage Closure)
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Norway
-
Denmark
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global structural heart devices market size was estimated at USD 6.33 billion in 2022 and is expected to reach USD 6.9 billion in 2023.
b. The global structural heart devices market is expected to grow at a compound annual growth rate of 9.3% from 2023 to 2030 to reach USD 12.8 billion by 2030.
b. North America dominated the structural heart devices market with a share of 40.8% in 2022. This is attributable to the increasing demand for minimally invasive procedures and the strategic presence of major players.
b. Some key players operating in the structural heart market include Boston Scientific Corporation; Medtronic; Edwards Lifesciences Corporation; Abbott; Biomerics; Comed BV; LivaNova PLC; JenaValve Technology, Inc.; and CardioKinetix
b. Key factors that are driving the market growth include rising prevalence of heart diseases and favoring health reimbursement policies.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





