- Home
- »
- Biotechnology
- »
-
Tissue Engineering Market Size, Share Analysis Report 2030GVR Report cover
![Tissue Engineering Market Size, Share & Trends Report]()
Tissue Engineering Market Size, Share & Trends Analysis Report By Application (Cord Blood & Cell Banking, Cancer, Orthopedics, Musculoskeletal & Spine, Dental, Urology, Cardiology & Vascular), By Region, And Segment Forecasts, 2023 - 2030
- Report ID: 978-1-68038-768-1
- Number of Pages: 110
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
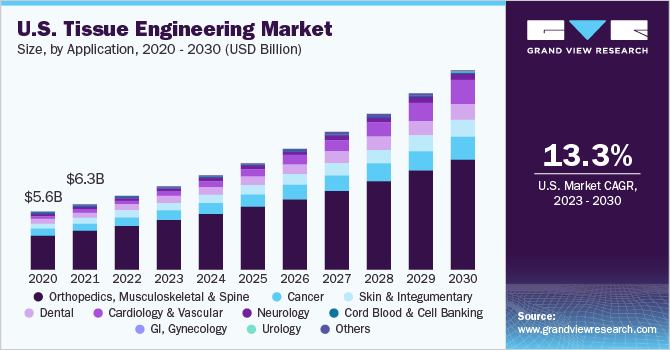
The global tissue engineering market size was estimated at USD 14.83 billion in 2022 and is anticipated to grow at a compound annual growth rate (CAGR) of 14.28% from 2023 to 2030. The growing incidence of chronic diseases, such as cardiovascular diseases, diabetes, and orthopedic disorders, has driven the demand for tissue engineering solutions capable of repairing or replacing damaged tissues. The field of tissue engineering has witnessed significant progress due to advances in biomaterials, 3D bioprinting, stem cell research, and gene editing techniques, which have enabled the development of complex and functional tissues.
According to a research study titled "Sexuality and relationship experiences of women with spinal cord injury: reflections from an Indian context", published in May 2022, it is estimated that approximately 250,000-500,000 people worldwide experience a spinal cord injury (SCI) annually. Also, according to the National Spinal Cord Injury Statistical Center, there are around 18,000 new cases of traumatic spinal cord injury (tSCI) in the U.S. each year.As chronic diseases are the main global causes of death and disability, they are anticipated to fuel market expansion throughout the forecast period.
Tissue regeneration technology is gaining popularity due to its effective products and low rejection rates. In addition, there is a growing trend of conducting more regeneration treatments. Currently, the ongoing pre-clinical research focuses on the utilization of tissue-engineered vascular grafts for cardiovascular surgery and treatment. Moreover, tissue-engineered bladders have shown successful implantation outside of the patient's body.
Technological advancements in the field of 3D tissue engineering, such as the replacement of embryo cells with stem cells, organ-on-a-chip technology, and the use of 3D bioprinters that can efficiently design in vitro implants, are expected to enhance growth. In addition, an increase in government funding for medical and academic research activities is anticipated to enhance the growth of the market for tissue engineering throughout the forecast period. For instance, in January 2023, Sartorius, a biopharmaceutical equipment supplier, has recently purchased a 10% stake in BICO, an innovative company specializing in 3D bioprinting. In addition to the USD 49.91 million share acquisition, both companies have revealed plans for a collaborative effort. They aim to work together on various research and development initiatives, with a focus on creating digital solutions for cell line development workflows.
Stem cell therapies hold considerable promise as treatments for various clinical conditions, leading to significant global investments in research and clinical translation. The rapid progress in stem cell research has contributed to enhanced disease management. Consequently, as the prevalence of cancer, diabetes, and other chronic disorders has increased, the focus on stem cell research has intensified.
The market is experiencing growth due to an increasing number of clinical studies conducted in the field of regenerative medicine and tissue engineering. According to the clinicaltrials.gov website, approximately 58 studies are being conducted related to regenerative medicine, and 63 studies focusing on tissue engineering. However, the market's progress is being hindered by challenges such as the high cost of product development and ethical concerns surrounding stem cell research and tissue-engineered products.
Application Insights
In terms of application, the orthopedics, musculoskeletal, and spine segment held the largest revenue share of 59.75% in 2022 owing to the increasing prevalence of musculoskeletal disorders. In addition, tissue engineering has emerged as a crucial therapy option for orthopedic surgeons in the management of several musculoskeletal disorders, ranging from meniscal deficits in young athletes to osteochondral abnormalities in the glenohumeral joint. According to WHO, rheumatoid arthritis affects more than 23 million people worldwide. Another study published in May 2021 showed that there were 460 cases of rheumatoid arthritis per 100,000 persons globally between 1980 and 2019.
The adoption of diverse growth strategies by market players coupled with the introduction of new products is projected to boost the segment expansion. In October 2022, the most cutting-edge allograft biologics for fusion was displayed by LifeNet Health during the North American Spine Society (NASS) 2022 Annual Meeting in Chicago. The display showcased ViviGen MIS, the first viable cellular allograft delivery device created especially for minimally invasive surgery.
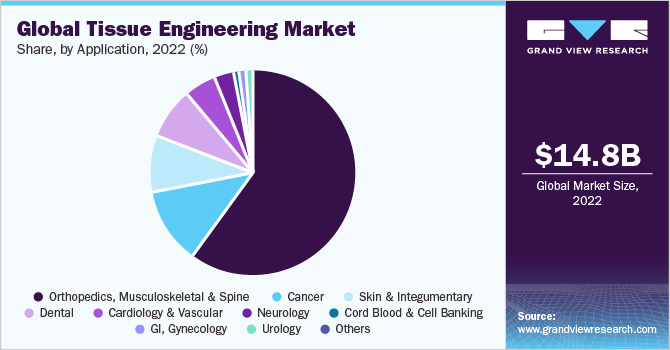
The cardiology & vascular segment is expected to register the highest CAGR of 25.9% over the forecast period owing to the rising prevalence of cardiovascular disorders. Notably, key players in the industry are actively engaged in developing stem cell therapies aimed at repairing, restoring, and re-vascularizing damaged heart tissues. In addition, research is being conducted on gene therapy, advanced biologics, and small molecules to stimulate the regeneration of damaged heart cells.
Regional Insights
In terms of region, North America dominated the market in 2022 and held the largest revenue share of 51.23% owing to rising awareness for stem cell therapy as well as the growing geriatric population and rising incidence of chronic diseases. Moreover, advanced technology for diagnosis and treatment of chronic disorders, availability of private and government funding, and high healthcare spending are some of the factors responsible for its high share.
Advancements in 3D tissue engineering technology and the presence of prominent market players are playing a significant role in driving growth through continuous product launches. For instance, in April 2022, Organogenesis, a leading company in regenerative medicine, presented the latest advancements in their wound care research, focusing on products such as PuraPly AM, Affinity, Apligraf, and Organogenesis Physician Solutions, at the 2022 Symposium on Advanced Wound Care Conference held in Phoenix, Arizona.
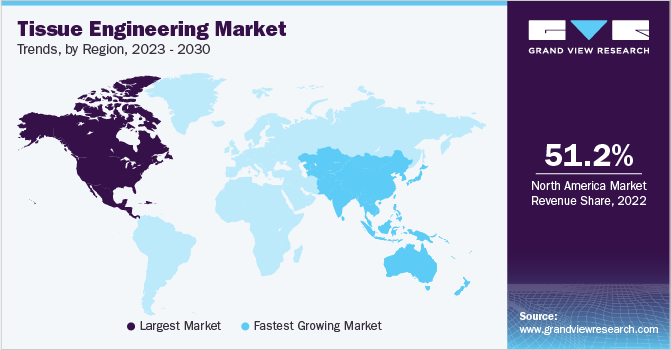
The Asia Pacific tissue engineering market is anticipated to witness the fastest CAGR of 15.84% during the forecast period. Japan is one of the leading countries to foster technological advances in the tissue engineering field. In addition, an increase in the prevalence of clinical disorders including cancer in Asian countries coupled with the evolution of 3D bioprinting and medical tourism are expected to propel the growth of the market in this region.
Key Companies & Market Share Insights
The market is highly competitive, with a large number of manufacturers vying for a majority of the market share. To sustain and expand their global presence, market participants employ essential business strategies such as product launches, approvals, strategic acquisitions, and continuous innovations. For instance, in January 2023, BioMed X and AbbVie extended their research partnership, as reported by the independent German biomedical research organization. The new U.S.-based research partnership will focus on immunology and tissue engineering after a first joint project on Alzheimer's disease at the BioMed X Institute in Heidelberg, Germany. Some prominent players in the global tissue engineering market include:
-
Zimmer Biomet Holdings, Inc.
-
AbbVie (Allergan)
-
Becton Dickinson and Company
-
B. Braun
-
Integra LifeSciences Corporation
-
Organogenesis Holdings Inc.
-
Medtronic
-
ACell, Inc.
-
Athersys, Inc.
-
Tissue Regenix Group plc
-
Stryker Corporation
-
RTI Surgical, Inc.
-
ReproCell, Inc.
-
Baxter International, Inc.
Tissue Engineering Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 16.94 billion
Revenue forecast in 2030
USD 43.13 billion
Growth rate
CAGR of 14.28% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
August 2023
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Application, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Key companies profiled
Zimmer Biomet Holdings Inc.; AbbVie (Allergan); Becton Dickinson and Company; B. Braun; Integra LifeSciences Corporation; Organogenesis Holdings Inc.; Medtronic; ACell, Inc.; Athersys, Inc.; Tissue Regenix Group plc; Stryker Corporation; RTI Surgical Inc.; ReproCell, Inc.; Baxter International, Inc.
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Tissue Engineering Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global tissue engineering market report based on application and region:
-
Application Outlook (Revenue, USD Billion, 2018 - 2030)
-
Cord Blood & Cell Banking
-
Cancer
-
GI, Gynecology
-
Dental
-
Skin & Integumentary
-
Urology
-
Orthopedics, Musculoskeletal, & Spine
-
Neurology
-
Cardiology & Vascular
-
Others
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Norway
-
Denmark
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global tissue engineering market size was estimated at USD 14.83 billion in 2022 and is expected to reach USD 16.94 billion in 2023.
b. The global tissue engineering market is expected to grow at a compound annual growth rate of 14.28% from 2023 to 2030 to reach USD 43.13 billion by 2030.
b. North America dominated the tissue engineering market with a share of 51.23% in 2022. This is attributable to a rise in awareness for stem cell therapy as well as a growing geriatric population in the region.
b. Some key players operating in the tissue engineering market include Medtronic plc; Zimmer Biomet Holdings, Inc; Allergan plc; Athersys, Inc; ACell, Inc.; Organogenesis Holdings Inc; Tissue Regenix Group plc; Stryker Corporation; RTI Surgical, Inc.; Integra LifeSciences Corporation; ReproCell, Inc.; and Baxter International, Inc.
b. Key factors that are driving the market growth include advancements in stem cell technology & tissue engineering, a rise in the number of clinical studies for regenerative medicine and tissue engineering, and increasing tissue engineering research funding.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





