- Home
- »
- Pharmaceuticals
- »
-
Biomarkers Market Size, Share And Growth Report, 2030GVR Report cover
![Biomarkers Market Size, Share & Trends Report]()
Biomarkers Market Size, Share & Trends Analysis Report By Type (Efficacy, Validation), By Product, By Application (Drug Discovery & Development, Personalized Medicine), By Disease, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: 978-1-68038-979-1
- Number of Pages: 150
- Format: Electronic (PDF)
- Historical Range: 2018 - 2022
- Industry: Healthcare
Biomarkers Market Size & Trends
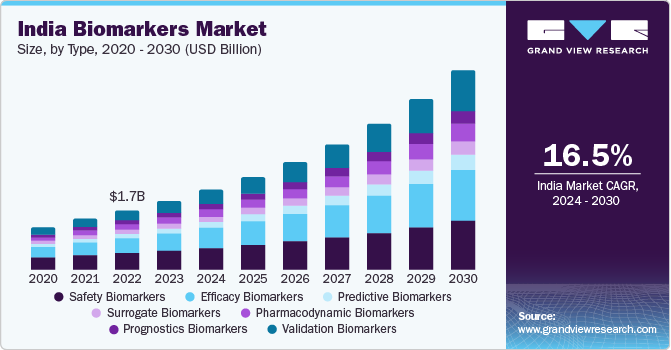
The global biomarkers market size was estimated at USD 81.04 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 13.36% from 2024 to 2030. The rising prevalence of cancer, significance of companion diagnostics, investments in research, and significant innovations owing to ongoing research are anticipated to drive market growth. For instance, in February 2023, National Institutes of Health announced funding of USD 4 million to Eastern Virginia Medical School (EVMS) for research and development of biomarker for early detection of aggressive prostate cancer.
Increase in the prevalence of fatal diseases has been observed over the past few years, which includes cancer, diabetes, cardiovascular disorders, and other chronic diseases. One of the major factors leading to an increase in their prevalence is lifestyle changes. According to the American Cancer Society, in 2022, an estimated 1.9 million new cancer cases are expected to be registered in the U.S., accounting for 609,360 deaths. Breast and lung cancers were observed as the most common ones worldwide. According to the World Health Organization (WHO), in 2022, an estimated 236,740 new cases of lung cancer were expected to be reported in the U.S., accounting for 130,180 deaths.
The use of biomarkers in infectious disease diagnosis is anticipated to become increasingly common in the upcoming years. For instance, as per Frontiers in Microbiology in 2020 , potential MicroRNA-based biomarkers have been identified for the diagnosis of infections such as influenza infections, rhinoviruses, HIV, tuberculosis, malaria, Ebola, and Hendra virus. These are aimed at facilitating early onset of infectious diseases. Biomarkers are also under study for the diagnosis of SARS-CoV-2. They have a high prognostic potential, which is projected to play a crucial role in the diagnosis of asymptomatic cases that hinder the tracking of pandemic cases. Furthermore, the high stability despite freeze and thaw cycles gives them an added advantage.
Innovative treatments that combine biomarkers with new or existing medicines are continuously being launched. For instance, biomarkers can now be used for the treatment of neurological diseases to track brain health by measuring molecules. Recent developments are making treatment of neurological diseases easier, for example, the development of biomarker signatures. This has resulted in noninvasive testing, faster drug development, and early diagnosis. R&D is leading to the discovery of novel biomarkers.
The emergence of digital biomarker which assists pharmaceutical companies with contextual and supplementary information to conclude clinical trial decisions is further propelling growth of the market. IXICO plc, a digital technology-based company that has expertise in neurosciences, is collaborating with biopharmaceutical companies to validate clinically digital biomarkers and use them in regulatory compliant clinical trials. Another new biomarker technology published in PLOS Journal in June 2021 is bowel cancer relapse detection biomarkers with the help of ctDNA. This can be used as a prognostic tool that predicts the recurrence with a 100% accuracy rate, thereby enabling better treatment.
Leading players are focusing on introducing programs that can increase the commercialization of biomarker-based products. For instance, in November 2022, NeoGenomics, under the sponsorship of ImmunoGen, launched a novel biomarker testing program for patients with Epithelial Ovarian Cancer (EOC). This initiative targeted FRα in patients with platinum-resistant EOC and increased patient access to FDA-approved ImmunoGen's ADC, ELAHERE. Similarly, in April 2021, Amgen launched a Biomarker Assist Program for metastatic Non-Small Cell Lung Cancer (NSCLC) patients and increased access to testing.
Market Concentration & Characteristics
Market growth stage is high and the pace is accelerating. The biomarkers market is characterized by a high degree of innovation fueled by rapid technological advancements. R&D is leading to the discovery of novel biomarkers. For instance, as per Nature Journal in 2020 , tetranectin is a potential biomarker for heart failure diagnosis. Similarly, in 2020, sTNFR2 was revealed as a novel biomarker for the diagnosis of acute adult T-cell leukemia/lymphoma. The market is likely to witness lucrative growth over the forecast period due to such discoveries and technological advancements.
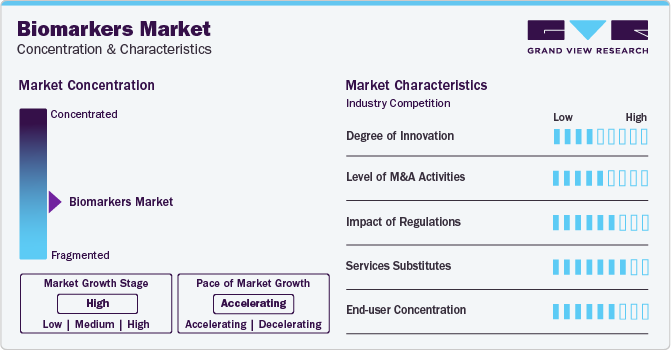
The industry is also characterized by a high level of partnership and collaboration activity by the leading players. It is one of the most adopted strategies by the players to enhance early commercialization of their products and improve the availability of products. For instance, in March 2023, Koneksa extended its partnership with SSI Strategy to scale and accelerate the adoption of digital biomarkers.
The biomarker segment is witnessing rapid technological advancements and high demand for personalized medicine. Thus, to ensure patient access to these new technologies, a reimbursement framework needs to be worked upon to meet the changing market scenario. This will potentially encourage manufacturers to invest in new products as reimbursement policies have a direct impact on the development and growth of diagnostic & prognostic tools.
The market players undertake this strategy to strengthen their product portfolios and offer diverse technologically advanced & innovative products to their customers. This is the most prominently adopted strategy by the companies to attract more customers. For instance, in July 2022, the U.S. FDA granted Breakthrough Device Designation to Elecsys Amyloid Plasma Panel for early detection of Alzheimer’s disease. Roche is the first IVD manufacturer to receive Breakthrough Device Designation for a blood-based biomarker test for Alzheimer’s.
Type Insights
The safety segment held the largest revenue share of 37.62% in 2023. Safety biomarkers can be used to customize therapies for patients, due to high risk of adverse reactions. They can predict or detect exposure effects or adverse drug events. Increase in the use of safety biomarkers in drug discovery & development is anticipated to boost market growth.Furthermore, an increase in population at high risk of developing various diseases, such as cancer, cardiovascular conditions, and kidney disorders, is expected to positively influence the market. Growing awareness of routine health checkups and lower drug attrition rates, which have been linked to biomarker-based therapies, are driving segment growth.
The efficacy biomarkers segment is expected to grow at the fastest CAGR from 2024 to 2030. Efficacy biomarkers aid in predicting patient responses to a specific drug. Despite the challenges in research, several findings have been reported on efficacy biomarkers. For instance, ATPase-copper Transporting β Polypeptide (ATP7B) is a biomarker used for detection of ovarian cancer. Furthermore, collaborative efforts between companies and academic institutes are expected to enhance the discovery of biomarkers. For instance, in June 2023, the National Cancer Institute announced the launch of ComboMatch platform trial which helps researchers to test efficacy of treatment combinations.
Product Insights
The consumables segment led the market in 2023. The growing emphasis on personalized and precision medicine has led to a growing need for biomarkers to identify specific disease markers and tailor treatment plans accordingly. This drives the demand for consumables used in biomarker discovery and validation. Moreover, the rising prevalence of chronic diseases such as cardiovascular diseases, cancer, and neurodegenerative disorders have led to an increase in consumption of consumables further driving market growth.
The services segment is anticipated to exhibit a lucrative CAGR over the projected period. Biomarkers are used in clinical trials for patient stratification, monitoring treatment responses, and assessing safety. Biomarker services support the design, implementation, and analysis of clinical trials, ensuring that biomarkers are effectively utilized for decision-making in drug development. Moreover, clinical laboratories and diagnostic service providers offer biomarker-based diagnostic testing services for various diseases. These services help in the accurate and reliable detection of biomarkers, contributing to early disease diagnosis and monitoring.
Application Insights
The drug discovery & development segment dominated the market in 2023. Biomarkers can be beneficial for accelerating drug development for certain diseases by predicting drug efficacy more easily than conventional clinical endpoints. Hence, they can help in identifying candidates that are likely to fail, thereby reducing drug development costs. Therefore, key players operating in this segment are focused on using biomarkers in drug development, which is leading to strategic alliances and thus driving the market.For instance, in June 2022, InterVenn Biosciences collaborated with the Foundation for the National Institutes of Health’s Biomarker Consortium and the Worldwide Innovative Network (WIN) Consortium, which aims to advance clinical trials, improve patient care, enhance precision oncology, and accelerate biomarker discovery.
The diagnostics segment is projected to register the fastest CAGR from 2024 to 2030. The increasing research focused on identification of new diagnostic biomarkers is fueling this growth. For instance, in February 2022, Japanese scientists found two new diagnostic tissue biomarkers, PHGDH and TRIM29, indicated for malignant pleural mesothelioma. These can be used to diagnose mesothelioma quickly and help doctors in differentiating between mesothelioma and other cancers.R&D in the segment confirms the development of novel biomarkers for early-stage detection of diseases such as Alzheimer's.
Disease Insights
The cancer segment led the market in 2023 and is expected to retain its dominance from 2024 to 2030. The growth of this segment is attributed to an increase in demand for rapid & accurate diagnostic tools and rise in global incidence of cancer. According to Global Cancer Observatory, about 19.3 million new cancer cases were recorded in 2020, while around 10 million cancer deaths were reported in the same year. In addition, growth in research activities for discovery and development of novel cancer biomarkers is widening the scope for market growth. In June 2022, researchers from the Tokyo University of Agriculture and Technology developed a novel technique based on DNA computation for the identification of cancer miRNA patterns. By using low concentrations of the target biomarkers, the new technique can be a promising tool for early cancer diagnosis.
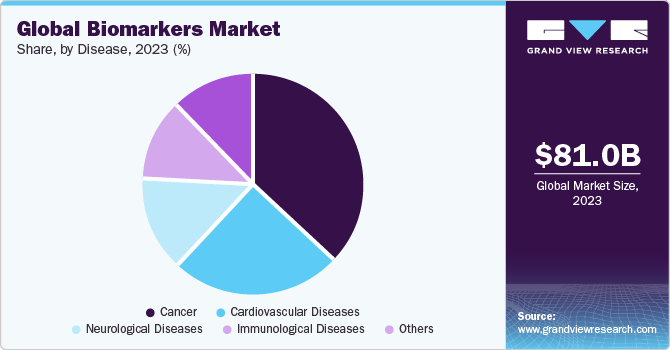
The immunological diseases segment is expected to attain the fastest CAGR from 2024 to 2030. This is mainly attributed to the increase in research aided with the rising prevalence of immunological diseases. For instance, in May 2023, according to article published by Boston Children’s Hospital, researchers have identified immune biomarkers that have helped in predicting COVID-19 severity and are mostly likely to help in future pandemics.Furthermore, increasing R&D in the renal biomarkers segment is anticipated to fuel the segment’s growth. For instance, in May 2022, researchers from the University of Houston, with the help of immunoproteomics-based discovery studies, reported potential biomarkers related to lupus nephritis related to clinical parameters, such as renal pathology indices. These biomarkers for lupus nephritis can be used to provide more reliable clinical blood tests for the disease, replacing kidney biopsy, which is a currently used invasive test.
Regional Insights
North America led the market with a revenue share of 43.94% in 2023, due to high disease burden, technological advancements, increased consumer awareness, supportive government initiatives, and improvements in healthcare infrastructure. Local presence of key players is anticipated to increase the penetration of novel biomarkers. Major players operating in the market include Abbott; Merck & Co., Inc.; Johnson & Johnson Services, Inc.; Thermo Fisher Scientific, Inc.; and Bio-Rad Laboratories, Inc.; Many academic institutions, research centers, and universities are collaborating with key market players to develop biomarkers for diagnosing and monitoring numerous diseases. Increasing efforts for the development of digital biomarkers and increasing investments as well as collaboration activities are anticipated to drive growth.
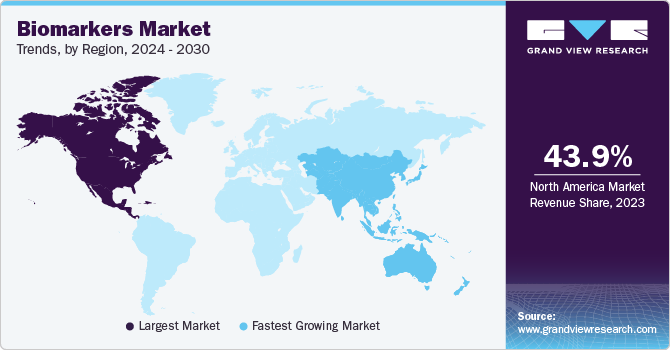
Asia Pacific is anticipated to attain the fastest CAGR from 2024 to 2030. Factors such as high prevalence of cancer, surge in funding for discovery, low cost of clinical trials in developing nations, and rising research initiatives are expected to support regional growth. Several advancements in R&D by biopharmaceutical companies are expected to positively impact the market. For instance, in December 2021, Denovo Biopharma LLC announced the discovery of a novel genetic marker for a gene therapy-based medicine. This would facilitate treatment for patients with recurrent high-grade glioma, an unmet medical need with estimated survival of less than a year.
Key Companies & Market Share Insights
Some of the leading market players include F. Hoffmann-La Roche AG, Abbott, QIAGEN, and PerkinElmer Incorporated. Key players are focusing on geographic expansions and gaining market approvals for innovative products. These players are heavily investing in advanced technology and infrastructure, allowing them to efficiently process & analyze a large volume of samples. Moreover, companies undertake various strategic initiatives with other companies and distributors to strengthen their market presence.
Atlas Genetics Ltd.; Hologic, Inc.;Myriad Genetics, Inc.; and Genomic Health, Inc. are some of the emerging market participants. These companies focus on achieving funding support from government bodies and healthcare organizations aided with novel product launches to capitalize on untapped avenues.
Key Biomarkers Companies:
- F. Hoffmann-La Roche AG
- Epigenomics AG
- Abbott
- Thermo Fisher Scientific Inc
- General Electric
- Eurofins Scientific
- Johnson & Johnson Services, Inc.
- QIAGEN
- Bio-Rad Laboratories, Inc.
- Siemens Healthineers AG
- Merck KGaA
- PerkinElmer Inc.
- Agilent Technologies, Inc.
Recent Developments
-
In October 2023, Labcorp announced the launch of tri-biomarkers blood test for diagnosis of Alzheimer’s disease.
-
In October 2023, Mindray announced the launch of high-sensitivity NT-proBNP and troponin I (hs-cTnI) cardiac biomarkers. The launch is expected to enhance the company’s product portfolio of cardiac biomarkers used in the management and diagnosis of cardiovascular diseases.
-
In August 2023 , Quest Diagnostics entered into partnership with Envision Sciences for the commercial launch of novel prostate cancer biomarker test for identification of severe and aggressive forms of the disease.
-
In February 2023, Cardio Diagnostics Holdings Inc. announced the launch of PrecisionCHD, a epigenetic-genetic blood test for early diagnosis of coronary heart disease.
Biomarkers Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 91.52 billion
Revenue forecast in 2030
USD 194.21 billion
Growth rate
CAGR of 13.36% from 2023 to 2030
Historical data
2023
Actual data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, product, application, disease, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; India; China; Japan; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; UAE; South Africa; Kuwait
Key companies profiled
F. Hoffmann-La Roche AG.; Abbott; Epigenomics AG; General Electric; Johnson & Johnson Services, Inc.; Thermo Fisher Scientific Inc.; Bio-Rad Laboratories, Inc.; Siemens Healthineers AG; QIAGEN; Merck KGaA; PerkinElmer Inc.; Agilent Technologies, Inc.; Eurofins Scientific
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Global Biomarkers Market Report Segmentation
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the biomarkers market report based on type, product, application, disease, and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Safety Biomarkers
-
Efficacy Biomarkers
-
Predictive Biomarkers
-
Surrogate Biomarkers
-
Pharmacodynamic Biomarkers
-
Prognostics Biomarkers
-
Validation Biomarkers
-
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Consumable
-
Services
-
Software
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Diagnostics
-
Drug Discovery & Development
-
Personalized Medicine
-
Disease Risk Assessment
-
Others
-
-
Disease Outlook (Revenue, USD Million, 2018 - 2030)
-
Cancer
-
Safety Biomarkers
-
Efficacy Biomarkers
-
Predictive Biomarkers
-
Surrogate Biomarkers
-
Pharmacodynamic Biomarkers
-
Prognostics Biomarkers
-
Validation Biomarkers
-
-
Cardiovascular Diseases
-
Safety Biomarkers
-
Efficacy Biomarkers
-
Predictive Biomarkers
-
Surrogate Biomarkers
-
Pharmacodynamic Biomarkers
-
Prognostics Biomarkers
-
Validation Biomarkers
-
-
Neurological Diseases
-
Safety Biomarkers
-
Efficacy Biomarkers
-
Predictive Biomarkers
-
Surrogate Biomarkers
-
Pharmacodynamic Biomarkers
-
Prognostics Biomarkers
-
Validation Biomarkers
-
-
Immunological Diseases
-
Safety Biomarkers
-
Efficacy Biomarkers
-
Predictive Biomarkers
-
Surrogate Biomarkers
-
Pharmacodynamic Biomarkers
-
Prognostics Biomarkers
-
Validation Biomarkers
-
-
Others
-
Safety Biomarkers
-
Efficacy Biomarkers
-
Predictive Biomarkers
-
Surrogate Biomarkers
-
Pharmacodynamic Biomarkers
-
Prognostics Biomarkers
-
Validation Biomarkers
-
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global biomarkers market size was valued at USD 81.04 billion in 2023 and is expected to reach USD 91.52 billion by 2024.
b. The global biomarkers market is expected to grow at a compound annual growth rate of 13.36% from 2024 to 2030 to reach USD 194.21 billion by 2030.
b. Cancer dominated the disease segment in 2023 with 37.45% of the market share, driven by a heightened demand for accurate and rapid diagnostic tools as well as a sharp growth in prevalence on a global scale.
b. Some key players operating in the biomarkers market include Abbott; Qiagen; F. Hoffmann-La Roche Ltd.; Thermo Fisher Scientific, Inc.; Siemens Healthineers AG; Bio-Rad Laboratories, Inc.; Johnson & Johnson Services, Inc; and Epigenomics AG.
b. The increasing prevalence of chronic diseases, advancements in the techniques used for the development of biomarker-based diagnostics, and the growing geriatric population are factors likely to boost the biomarkers market significantly throughout the forecast period.
Table of Contents
Chapter 1. Methodology and Scope
1.1. Market Segmentation & Scope
1.2. Segment Definitions
1.2.1. Type
1.2.2. Product
1.2.3. Application
1.2.4. Disease
1.2.5. Regional scope
1.2.6. Estimates and forecasts timeline
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased database
1.4.2. GVR’s internal database
1.4.3. Secondary sources
1.4.4. Primary research
1.4.5. Details of primary research
1.4.5.1. Data for Primary Interviews in North America
1.4.5.2. Data for Primary Interviews in Europe
1.4.5.3. Data for Primary Interviews in Asia Pacific
1.4.5.4. Data for Primary Interviews in Latin America
1.4.5.5. Data for Primary Interviews in MEA
1.5. Information or Data Analysis
1.5.1. Data analysis models
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.2. Approach 1: Commodity flow approach
1.7.3. Volume price analysis (Model 2)
1.7.4. Approach 2: Volume price analysis
1.8. List of Secondary Sources
1.9. List of Primary Sources
1.10. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.2.1. Type and product outlook
2.2.2. Application and disease outlook
2.2.3. Regional outlook
2.3. Competitive Insights
Chapter 3. Biomarkers Market Variables, Trends & Scope
3.1. Market Lineage Outlook
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook
3.2. Market Dynamics
3.2.1. Market driver analysis
3.2.1.1. Increasing funding for biomarkers
3.2.1.2. Increasing prevalence for chronic diseases
3.2.1.3. Technological advancement
3.2.1.4. Growing importance of companion diagnostics
3.2.2. Market restraint analysis
3.2.2.1. Lack of reimbursement policies for biomarkers
3.2.2.2. High capital investment and lengthy timelines for biomarker development
3.2.3. Market opportunity analysis
3.2.3.1. Emergence of personalized medicine
3.2.3.2. Emerging economies
3.2.4. Market challenge analysis
3.2.4.1. Challenges associated with biomarkers validation
3.2.4.2. Technical issues related to sample collection and storage
3.3. Biomarkers Market Analysis Tools
3.3.1. Industry Analysis - Porter’s
3.3.1.1. Supplier power
3.3.1.2. Buyer power
3.3.1.3. Substitution threat
3.3.1.4. Threat of new entrant
3.3.1.5. Competitive rivalry
3.3.2. PESTEL Analysis
3.3.2.1. Political landscape
3.3.2.2. Technological landscape
3.3.2.3. Economic landscape
3.3.3. Pricing Analysis
3.3.3.1. Selling price of biomarker by top players
Chapter 4. Biomarkers Market: Type Estimates & Trend Analysis
4.1. Type Market Share, 2023 & 2030
4.2. Segment Dashboard
4.3. Global Biomarkers Market by Type Outlook
4.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
4.4.1. Safety biomarkers
4.4.1.1. Safety biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.2. Efficacy biomarkers
4.4.2.1. Efficacy biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.3. Predictive biomarkers
4.4.3.1. Predictive biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.4. Surrogate biomarkers
4.4.4.1. Surrogate biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.5. Pharmacodynamic biomarkers
4.4.5.1. Pharmacodynamic Biomarkers Market Estimates and forecasts, 2018 to 2030 (USD Million)
4.4.6. Prognostics biomarkers
4.4.6.1. Prognostics biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.7. Validation biomarkers
4.4.7.1. Validation biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
Chapter 5. Biomarkers Market: Product Estimates & Trend Analysis
5.1. Product Market Share, 2023 & 2030
5.2. Segment Dashboard
5.3. Global Biomarkers Market by Product Outlook
5.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
5.4.1. Consumables
5.4.1.1. Consumables market estimates and forecasts, 2018 to 2030 (USD Million)
5.4.2. Services
5.4.2.1. Services market estimates and forecasts, 2018 to 2030 (USD Million)
5.4.3. Software
5.4.3.1. Software market estimates and forecasts, 2018 to 2030 (USD Million)
Chapter 6. Biomarkers Market: Application Estimates & Trend Analysis
6.1. Application Market Share, 2023 & 2030
6.2. Segment Dashboard
6.3. Global Biomarkers Market by Application Outlook
6.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
6.4.1. Diagnostics
6.4.1.1. Diagnostics market estimates and forecasts, 2018 to 2030 (USD Million)
6.4.2. Drug Discovery and Development
6.4.2.1. Drug discovery and development market estimates and forecasts, 2018 to 2030 (USD Million)
6.4.3. Personalized Medicine
6.4.3.1. Personalized medicine market estimates and forecasts, 2018 to 2030 (USD Million)
6.4.4. Disease Risk Assessment
6.4.4.1. Disease risk assessment market estimates and forecasts, 2018 to 2030 (USD Million)
6.4.5. Others
6.4.5.1. Others market estimates and forecasts, 2018 to 2030 (USD Million)
Chapter 7. Biomarkers Market: Disease Estimates & Trend Analysis
7.1. Disease Market Share, 2023 & 2030
7.2. Segment Dashboard
7.3. Global Biomarkers Market by Disease Outlook
7.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
7.4.1. Cancer
7.4.1.1. Cancer market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.1.2. Safety biomarkers
7.4.1.2.1. Safety biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.1.3. Efficacy biomarkers
7.4.1.3.1. Efficacy biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.1.4. Predictive biomarkers
7.4.1.4.1. Predictive biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.1.5. Surrogate biomarkers
7.4.1.5.1. Surrogate biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.1.6. Pharmacodynamic biomarkers
7.4.1.6.1. Pharmacodynamic Biomarkers Market Estimates and forecasts, 2018 to 2030 (USD Million)
7.4.1.7. Prognostics biomarkers
7.4.1.7.1. Prognostics biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.1.8. Validation biomarkers
7.4.1.8.1. Validation biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.2. Cardiovascular Diseases
7.4.2.1. Cardiovascular diseases market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.2.2. Safety biomarkers
7.4.2.2.1. Safety biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.2.3. Efficacy biomarkers
7.4.2.3.1. Efficacy biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.2.4. Predictive biomarkers
7.4.2.4.1. Predictive biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.2.5. Surrogate biomarkers
7.4.2.5.1. Surrogate biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.2.6. Pharmacodynamic biomarkers
7.4.2.6.1. Pharmacodynamic Biomarkers Market Estimates and forecasts, 2018 to 2030 (USD Million)
7.4.2.7. Prognostics biomarkers
7.4.2.7.1. Prognostics biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.2.8. Validation biomarkers
7.4.2.8.1. Validation biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)Neurological Diseases
7.4.3.1. Neurological diseases market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.3.2. Safety biomarkers
7.4.3.2.1. Safety biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.3.3. Efficacy biomarkers
7.4.3.3.1. Efficacy biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.3.4. Predictive biomarkers
7.4.3.4.1. Predictive biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.3.5. Surrogate biomarkers
7.4.3.5.1. Surrogate biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.3.6. Pharmacodynamic biomarkers
7.4.3.6.1. Pharmacodynamic Biomarkers Market Estimates and forecasts, 2018 to 2030 (USD Million)
7.4.3.7. Prognostics biomarkers
7.4.3.7.1. Prognostics biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.3.8. Validation biomarkers
7.4.3.8.1. Validation biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.4. Immunological Diseases
7.4.4.1. Immunological diseases market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.4.2. Safety biomarkers
7.4.4.2.1. Safety biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.4.3. Efficacy biomarkers
7.4.4.3.1. Efficacy biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.4.4. Predictive biomarkers
7.4.4.4.1. Predictive biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.4.5. Surrogate biomarkers
7.4.4.5.1. Surrogate biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.4.6. Pharmacodynamic biomarkers
7.4.4.6.1. Pharmacodynamic Biomarkers Market Estimates and forecasts, 2018 to 2030 (USD Million)
7.4.4.7. Prognostics biomarkers
7.4.4.7.1. Prognostics biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.4.8. Validation biomarkers
7.4.4.8.1. Validation biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.5. Others
7.4.5.1. Others market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.5.2. Safety biomarkers
7.4.5.2.1. Safety biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.5.3. Efficacy biomarkers
7.4.5.3.1. Efficacy biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.5.4. Predictive biomarkers
7.4.5.4.1. Predictive biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.5.5. Surrogate biomarkers
7.4.5.5.1. Surrogate biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.5.6. Pharmacodynamic biomarkers
7.4.5.6.1. Pharmacodynamic Biomarkers Market Estimates and forecasts, 2018 to 2030 (USD Million)
7.4.5.7. Prognostics biomarkers
7.4.5.7.1. Prognostics biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
7.4.5.8. Validation biomarkers
7.4.5.8.1. Validation biomarkers market estimates and forecasts, 2018 to 2030 (USD Million)
Chapter 8. Biomarkers Market: Regional Estimates & Trend Analysis
8.1. Regional Market Share Analysis, 2023 & 2030
8.2. Regional Market Dashboard
8.3. Global Regional Market Snapshot
8.4. Market Size, & Forecasts Trend Analysis, 2018 to 2030:
8.5. North America
8.5.1. U.S.
8.5.1.1. Key country dynamics
8.5.1.2. Regulatory framework/ reimbursement structure
8.5.1.3. Competitive scenario
8.5.1.4. U.S. market estimates and forecasts, 2018 to 2030 (USD Million)
8.5.2. Canada
8.5.2.1. Key country dynamics
8.5.2.2. Regulatory framework/ reimbursement structure
8.5.2.3. Competitive scenario
8.5.2.4. Canada market estimates and forecasts, 2018 to 2030 (USD Million)
8.6. Europe
8.6.1. UK
8.6.1.1. Key country dynamics
8.6.1.2. Regulatory framework/ reimbursement structure
8.6.1.3. Competitive scenario
8.6.1.4. UK market estimates and forecasts, 2018 to 2030 (USD Million)
8.6.2. Germany
8.6.2.1. Key country dynamics
8.6.2.2. Regulatory framework/ reimbursement structure
8.6.2.3. Competitive scenario
8.6.2.4. Germany market estimates and forecasts, 2018 to 2030 (USD Million)
8.6.3. France
8.6.3.1. Key country dynamics
8.6.3.2. Regulatory framework/ reimbursement structure
8.6.3.3. Competitive scenario
8.6.3.4. France market estimates and forecasts, 2018 to 2030 (USD Million)
8.6.4. Italy
8.6.4.1. Key country dynamics
8.6.4.2. Regulatory framework/ reimbursement structure
8.6.4.3. Competitive scenario
8.6.4.4. Italy market estimates and forecasts, 2018 to 2030 (USD Million)
8.6.5. Spain
8.6.5.1. Key country dynamics
8.6.5.2. Regulatory framework/ reimbursement structure
8.6.5.3. Competitive scenario
8.6.5.4. Spain market estimates and forecasts, 2018 to 2030 (USD Million)
8.6.6. Norway
8.6.6.1. Key country dynamics
8.6.6.2. Regulatory framework/ reimbursement structure
8.6.6.3. Competitive scenario
8.6.6.4. Norway market estimates and forecasts, 2018 to 2030 (USD Million)
8.6.7. Sweden
8.6.7.1. Key country dynamics
8.6.7.2. Regulatory framework/ reimbursement structure
8.6.7.3. Competitive scenario
8.6.7.4. Sweden market estimates and forecasts, 2018 to 2030 (USD Million)
8.6.8. Denmark
8.6.8.1. Key country dynamics
8.6.8.2. Regulatory framework/ reimbursement structure
8.6.8.3. Competitive scenario
8.6.8.4. Denmark market estimates and forecasts, 2018 to 2030 (USD Million)
8.7. Asia Pacific
8.7.1. Japan
8.7.1.1. Key country dynamics
8.7.1.2. Regulatory framework/ reimbursement structure
8.7.1.3. Competitive scenario
8.7.1.4. Japan market estimates and forecasts, 2018 to 2030 (USD Million)
8.7.2. China
8.7.2.1. Key country dynamics
8.7.2.2. Regulatory framework/ reimbursement structure
8.7.2.3. Competitive scenario
8.7.2.4. China market estimates and forecasts, 2018 to 2030 (USD Million)
8.7.3. India
8.7.3.1. Key country dynamics
8.7.3.2. Regulatory framework/ reimbursement structure
8.7.3.3. Competitive scenario
8.7.3.4. India market estimates and forecasts, 2018 to 2030 (USD Million)
8.7.4. Australia
8.7.4.1. Key country dynamics
8.7.4.2. Regulatory framework/ reimbursement structure
8.7.4.3. Competitive scenario
8.7.4.4. Australia market estimates and forecasts, 2018 to 2030 (USD Million)
8.7.5. South Korea
8.7.5.1. Key country dynamics
8.7.5.2. Regulatory framework/ reimbursement structure
8.7.5.3. Competitive scenario
8.7.5.4. South Korea market estimates and forecasts, 2018 to 2030 (USD Million)
8.7.6. Thailand
8.7.6.1. Key country dynamics
8.7.6.2. Regulatory framework/ reimbursement structure
8.7.6.3. Competitive scenario
8.7.6.4. Thailand market estimates and forecasts, 2018 to 2030 (USD Million)
8.8. Latin America
8.8.1. Brazil
8.8.1.1. Key country dynamics
8.8.1.2. Regulatory framework/ reimbursement structure
8.8.1.3. Competitive scenario
8.8.1.4. Brazil market estimates and forecasts, 2018 to 2030 (USD Million)
8.8.2. Mexico
8.8.2.1. Key country dynamics
8.8.2.2. Regulatory framework/ reimbursement structure
8.8.2.3. Competitive scenario
8.8.2.4. Mexico market estimates and forecasts, 2018 to 2030 (USD Million)
8.8.3. Argentina
8.8.3.1. Key country dynamics
8.8.3.2. Regulatory framework/ reimbursement structure
8.8.3.3. Competitive scenario
8.8.3.4. Argentina market estimates and forecasts, 2018 to 2030 (USD Million)
8.9. MEA
8.9.1. South Africa
8.9.1.1. Key country dynamics
8.9.1.2. Regulatory framework/ reimbursement structure
8.9.1.3. Competitive scenario
8.9.1.4. South Africa market estimates and forecasts, 2018 to 2030 (USD Million)
8.9.2. Saudi Arabia
8.9.2.1. Key Country Dynamics
8.9.2.2. Regulatory framework/ reimbursement structure
8.9.2.3. Competitive scenario
8.9.2.4. Saudi Arabia market estimates and forecasts for 2018 to 2030 (USD Million)
8.9.3. UAE
8.9.3.1. Key country dynamics
8.9.3.2. Regulatory framework/ reimbursement structure
8.9.3.3. Competitive scenario
8.9.3.4. UAE market estimates and forecasts, 2018 to 2030 (USD Million)
8.9.4. Kuwait
8.9.4.1. Key country dynamics
8.9.4.2. Regulatory framework/ reimbursement structure
8.9.4.3. Competitive scenario
8.9.4.4. Kuwait market estimates and forecasts, 2018 to 2030 (USD Million)
Chapter 9. Competitive Landscape
9.1. Recent Developments & Impact Analysis, By Key Market Participants
9.2. Company/Competition Categorization
9.3. Vendor Landscape
9.3.1. List of key distributors and channel partners
9.3.2. Key customers
9.3.3. Key company market share analysis, 2023
9.3.4. F. Hoffmann-La Roche Ltd.
9.3.4.1. Company overview
9.3.4.2. Financial performance
9.3.4.3. Product benchmarking
9.3.4.4. Strategic initiatives
9.3.5. Abbott
9.3.5.1. Company overview
9.3.5.2. Financial performance
9.3.5.3. Product benchmarking
9.3.5.4. Strategic initiatives
9.3.6. Epigenomics AG
9.3.6.1. Company overview
9.3.6.2. Financial performance
9.3.6.3. Product benchmarking
9.3.6.4. Strategic initiatives
9.3.7. General Electric
9.3.7.1. Company overview
9.3.7.2. Financial performance
9.3.7.3. Product benchmarking
9.3.7.4. Strategic initiatives
9.3.8. Johnson & Johnson Services, Inc.
9.3.8.1. Company overview
9.3.8.2. Financial performance
9.3.8.3. Product benchmarking
9.3.8.4. Strategic initiatives
9.3.9. Thermo Fisher Scientific Inc.
9.3.9.1. Company overview
9.3.9.2. Financial performance
9.3.9.3. Product benchmarking
9.3.9.4. Strategic initiatives
9.3.10. Bio-Rad Laboratories, Inc.
9.3.10.1. Company overview
9.3.10.2. Financial performance
9.3.10.3. Product benchmarking
9.3.10.4. Strategic initiatives
9.3.11. Siemens Healthineers AG
9.3.11.1. Company overview
9.3.11.2. Financial performance
9.3.11.3. Product benchmarking
9.3.11.4. Strategic initiatives
9.3.12. QIAGEN
9.3.12.1. Company overview
9.3.12.2. Financial performance
9.3.12.3. Product benchmarking
9.3.12.4. Strategic initiatives
9.3.13. Merck KGaA
9.3.13.1. Company overview
9.3.13.2. Financial performance
9.3.13.3. Product benchmarking
9.3.13.4. Strategic initiatives
9.3.14. PerkinElmer Inc.
9.3.14.1. Company overview
9.3.14.2. Financial performance
9.3.14.3. Product benchmarking
9.3.14.4. Strategic initiatives
9.3.15. Agilent Technologies, Inc.
9.3.15.1. Company overview
9.3.15.2. Financial performance
9.3.15.3. Product benchmarking
9.3.15.4. Strategic initiatives
9.3.16. Eurofins Scientific
9.3.16.1. Company overview
9.3.16.2. Financial performance
9.3.16.3. Product benchmarking
9.3.16.4. Strategic initiatives
List of Tables
Table 1 List of Secondary Sources
Table 2 List of Abbreviations
Table 3 North America Biomarkers Market, By Country, 2018 - 2030 (USD Million)
Table 4 North America Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 5 North America Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 6 North America Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 7 North America Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 8 U.S. Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 9 U.S. Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 10 U.S. Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 11 U.S. Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 12 Canada Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 13 Canada Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 14 Canada Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 15 Canada Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 16 Europe Biomarkers Market, By Country, 2018 - 2030 (USD Million)
Table 17 Europe Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 18 Europe Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 19 Europe Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 20 Europe Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 21 Germany Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 22 Germany Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 23 Germany Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 24 Germany Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 25 UK Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 26 UK Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 27 UK Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 28 UK Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 29 France Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 30 France Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 31 France Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 32 France Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 33 Spain Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 34 Spain Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 35 Spain Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 36 Spain Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 37 Italy Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 38 Italy Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 39 Italy Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 40 Italy Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 41 Denmark Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 42 Denmark Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 43 Denmark Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 44 Denmark Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 45 Norway Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 46 Norway Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 47 Norway Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 48 Norway Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 49 Sweden Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 50 Sweden Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 51 Sweden Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 52 Sweden Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 53 Asia Pacific Biomarkers Market, By Country, 2018 - 2030 (USD Million)
Table 54 Asia Pacific Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 55 Asia Pacific Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 56 Asia Pacific Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 57 Asia Pacific Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 58 Japan Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 59 Japan Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 60 Japan Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 61 Japan Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 62 China Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 63 China Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 64 China Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 65 China Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 66 India Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 67 India Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 68 India Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 69 India Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 70 South Korea Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 71 South Korea Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 72 South Korea Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 73 South Korea Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 74 Australia Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 75 Australia Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 76 Australia Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 77 Australia Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 78 Thailand Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 79 Thailand Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 80 Thailand Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 81 Thailand Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 82 Latin America Biomarkers Market, By Country, 2018 - 2030 (USD Million)
Table 83 Latin America Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 84 Latin America Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 85 Latin America Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 86 Latin America Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 87 Brazil Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 88 Brazil Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 89 Brazil Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 90 Brazil Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 91 Argentina Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 92 Argentina Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 93 Argentina Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 94 Argentina Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 95 Mexico Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 96 Mexico Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 97 Mexico Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 98 Mexico Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 99 Middle East and Africa Biomarkers Market, By Country, 2018 - 2030 (USD Million)
Table 100 Middle East and Africa Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 101 Middle East and Africa Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 102 Middle East and Africa Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 103 Middle East and Africa Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 104 South Africa Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 105 South Africa Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 106 South Africa Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 107 South Africa Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 108 Saudi Arabia Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 109 Saudi Arabia Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 110 Saudi Arabia Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 111 Saudi Arabia Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 112 Kuwait Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 113 Kuwait Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 114 Kuwait Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 115 Kuwait Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
Table 116 UAE Biomarkers Market, By Type, 2018 - 2030 (USD Million)
Table 117 UAE Biomarkers Market, By Product, 2018 - 2030 (USD Million)
Table 118 UAE Biomarkers Market, By Application, 2018 - 2030 (USD Million)
Table 119 UAE Biomarkers Market, By Disease, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Market research process
Fig. 2 Data triangulation techniques
Fig. 3 Primary research pattern
Fig. 4 Primary Interviews in North America
Fig. 5 Primary Interviews in Europe
Fig. 6 Primary Interviews in APAC
Fig. 7 Primary Interviews in Latin America
Fig. 8 Primary Interviews in MEA
Fig. 9 Market research approaches
Fig. 10 Value-chain-based sizing & forecasting
Fig. 11 QFD modeling for market share assessment
Fig. 12 Market formulation & validation
Fig. 13 Biomarkers market: market outlook
Fig. 14 Tumor ablation competitive insights
Fig. 15 Parent market outlook
Fig. 16 Related/ancillary market outlook
Fig. 17 Penetration and growth prospect mapping
Fig. 18 Industry value chain analysis
Fig. 19 Biomarkers market driver impact
Fig. 20 Biomarkers market restraint impact
Fig. 21 Biomarkers market strategic initiatives analysis
Fig. 22 Biomarkers market: Type movement analysis
Fig. 23 Biomarkers market: Type outlook and key takeaways
Fig. 24 Safety biomarkers market estimates and forecast, 2018 - 2030
Fig. 25 Efficacy biomarkers agents estimates and forecast, 2018 - 2030
Fig. 26 Predictive biomarkers market estimates and forecast, 2018 - 2030
Fig. 27 Surrogate biomarkers estimates and forecast, 2018 - 2030
Fig. 28 Pharmacodynamic biomarkers market estimates and forecast, 2018 - 2030
Fig. 29 Prognostics biomarkers estimates and forecast, 2018 - 2030
Fig. 30 Validation biomarkers estimates and forecast, 2018 - 2030
Fig. 31 Biomarkers market: Product movement analysis
Fig. 32 Biomarkers market: Product outlook and key takeaways
Fig. 33 Consumables market estimates and forecast, 2018 - 2030
Fig. 34 Services estimates and forecast, 2018 - 2030
Fig. 35 Software market estimates and forecast, 2018 - 2030
Fig. 36 Biomarkers market: Disease movement analysis
Fig. 37 Biomarkers market: Disease outlook and key takeaways
Fig. 38 Diagnostics market estimates and forecast, 2018 - 2030
Fig. 39 Drug discovery and development estimates and forecast, 2018 - 2030
Fig. 40 Personalized medicine market estimates and forecast, 2018 - 2030
Fig. 41 Disease risk assessment estimates and forecast, 2018 - 2030
Fig. 42 Others market estimates and forecast, 2018 - 2030
Fig. 43 Biomarkers market: Disease movement analysis
Fig. 44 Biomarkers market: Disease outlook and key takeaways
Fig. 45 Cancer market estimates and forecast, 2018 - 2030
Fig. 46 Cardiovascular diseases estimates and forecast, 2018 - 2030
Fig. 47 Neurological diseases market estimates and forecast, 2018 - 2030
Fig. 48 Immunological diseases estimates and forecast, 2018 - 2030
Fig. 49 Others market estimates and forecast, 2018 - 2030
Fig. 50 Global ophthalmic drugs market: Regional movement analysis
Fig. 51 Global ophthalmic drugs market: Regional outlook and key takeaways
Fig. 52 Global ophthalmic drugs market share and leading players
Fig. 53 North America market share and leading players
Fig. 54 Europe market share and leading players
Fig. 55 Asia Pacific market share and leading players
Fig. 56 Latin America market share and leading players
Fig. 57 Middle East & Africa market share and leading players
Fig. 58 North America: SWOT
Fig. 59 Europe SWOT
Fig. 60 Asia Pacific SWOT
Fig. 61 Latin America SWOT
Fig. 62 MEA SWOT
Fig. 63 North America, by country
Fig. 64 North America
Fig. 65 North America market estimates and forecasts, 2018 - 2030
Fig. 66 U.S. key country dynamics
Fig. 67 U.S. market estimates and forecasts, 2018 - 2030
Fig. 68 Canada key country dynamics
Fig. 69 Canada market estimates and forecasts, 2018 - 2030
Fig. 70 Europe
Fig. 71 Europe market estimates and forecasts, 2018 - 2030
Fig. 72 UK key country dynamics
Fig. 73 UK market estimates and forecasts, 2018 - 2030
Fig. 74 Germany key country dynamics
Fig. 75 Germany market estimates and forecasts, 2018 - 2030
Fig. 76 France key country dynamics
Fig. 77 France market estimates and forecasts, 2018 - 2030
Fig. 78 Italy key country dynamics
Fig. 79 Italy market estimates and forecasts, 2018 - 2030
Fig. 80 Spain key country dynamics
Fig. 81 Spain market estimates and forecasts, 2018 - 2030
Fig. 82 Denmark key country dynamics
Fig. 83 Denmark market estimates and forecasts, 2018 - 2030
Fig. 84 Sweden key country dynamics
Fig. 85 Sweden market estimates and forecasts, 2018 - 2030
Fig. 86 Norway key country dynamics
Fig. 87 Norway market estimates and forecasts, 2018 - 2030
Fig. 88 Asia Pacific
Fig. 89 Asia Pacific market estimates and forecasts, 2018 - 2030
Fig. 90 China key country dynamics
Fig. 91 China market estimates and forecasts, 2018 - 2030
Fig. 92 Japan key country dynamics
Fig. 93 Japan market estimates and forecasts, 2018 - 2030
Fig. 94 India key country dynamics
Fig. 95 India market estimates and forecasts, 2018 - 2030
Fig. 96 Thailand key country dynamics
Fig. 97 Thailand market estimates and forecasts, 2018 - 2030
Fig. 98 South Korea key country dynamics
Fig. 99 South Korea market estimates and forecasts, 2018 - 2030
Fig. 100 Australia key country dynamics
Fig. 101 Australia market estimates and forecasts, 2018 - 2030
Fig. 102 Latin America
Fig. 103 Latin America market estimates and forecasts, 2018 - 2030
Fig. 104 Brazil key country dynamics
Fig. 105 Brazil market estimates and forecasts, 2018 - 2030
Fig. 106 Mexico key country dynamics
Fig. 107 Mexico market estimates and forecasts, 2018 - 2030
Fig. 108 Argentina key country dynamics
Fig. 109 Argentina market estimates and forecasts, 2018 - 2030
Fig. 110 Middle East and Africa
Fig. 111 Middle East and Africa market estimates and forecasts, 2018 - 2030
Fig. 112 South Africa key country dynamics
Fig. 113 South Africa market estimates and forecasts, 2018 - 2030
Fig. 114 Saudi Arabia key country dynamics
Fig. 115 Saudi Arabia market estimates and forecasts, 2018 - 2030
Fig. 116 UAE key country dynamics
Fig. 117 UAE market estimates and forecasts, 2018 - 2030
Fig. 118 Kuwait key country dynamics
Fig. 119 Kuwait market estimates and forecasts, 2018 - 2030
Fig. 120 Market share of key market players- Biomarkers marketWhat questions do you have? Get quick response from our industry experts. Request a Free ConsultationMarket Segmentation
- Biomarkers Type Outlook (Revenue, USD Million, 2018 - 2030)
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Biomarkers Product Outlook (Revenue, USD Million, 2018 - 2030)
- Consumables
- Services
- Software
- Biomarkers Application Outlook (Revenue, USD Million, 2018 - 2030)
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Biomarkers Disease Outlook (Revenue, USD Million, 2018 - 2030)
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Biomarkers Regional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- North America Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- North America Biomarkers Market, By Product
- Consumables
- Services
- Software
- North America Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- North America Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- U.S.
- U.S. Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- U.S. Biomarkers Market, By Product
- Consumables
- Services
- Software
- U.S. Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- U.S. Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- U.S. Biomarkers Market, By Type
- Canada
- Canada Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Canada Biomarkers Market, By Product
- Consumables
- Services
- Software
- Canada Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Canada Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Canada Biomarkers Market, By Type
- North America Biomarkers Market, By Type
- Europe
- Europe Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Europe Biomarkers Market, By Product
- Consumables
- Services
- Software
- Europe Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Europe Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- UK
- UK Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- UK Biomarkers Market, By Product
- Consumables
- Services
- Software
- UK Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- UK Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- UK Biomarkers Market, By Type
- Germany
- Germany Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Germany Biomarkers Market, By Product
- Consumables
- Services
- Software
- Germany Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Germany Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Germany Biomarkers Market, By Type
- France
- France Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- France Biomarkers Market, By Product
- Consumables
- Services
- Software
- France Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- France Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- France Biomarkers Market, By Type
- Italy
- Italy Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Italy Biomarkers Market, By Product
- Consumables
- Services
- Software
- Italy Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Italy Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Italy Biomarkers Market, By Type
- Spain
- Spain Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Spain Biomarkers Market, By Product
- Consumables
- Services
- Software
- Spain Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Spain Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Spain Biomarkers Market, By Type
- Denmark
- Denmark Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Denmark Biomarkers Market, By Product
- Consumables
- Services
- Software
- DenmarkBiomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- DenmarkBiomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Denmark Biomarkers Market, By Type
- Sweden
- Sweden Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Sweden Biomarkers Market, By Product
- Consumables
- Services
- Software
- Sweden Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Sweden Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Sweden Biomarkers Market, By Type
- Norway
- Norway Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Norway Biomarkers Market, By Product
- Consumables
- Services
- Software
- Norway Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Norway Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Norway Biomarkers Market, By Type
- Europe Biomarkers Market, By Type
- Asia Pacific
- Asia Pacific Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Asia Pacific Biomarkers Market, By Product
- Consumables
- Services
- Software
- Asia Pacific Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Asia Pacific Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Japan
- Japan Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Japan Biomarkers Market, By Product
- Consumables
- Services
- Software
- Japan Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Japan Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Japan Biomarkers Market, By Type
- China
- China Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- China Biomarkers Market, By Product
- Consumables
- Services
- Software
- China Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- China Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- China Biomarkers Market, By Type
- India
- India Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- India Biomarkers Market, By Product
- Consumables
- Services
- Software
- India Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- India Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- India Biomarkers Market, By Type
- South Korea
- South Korea Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- South Korea Biomarkers Market, By Product
- Consumables
- Services
- Software
- South Korea Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- South Korea Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- South Korea Biomarkers Market, By Type
- Australia
- Australia Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Australia Biomarkers Market, By Product
- Consumables
- Services
- Software
- Australia Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Australia Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Australia Biomarkers Market, By Type
- Thailand
- Thailand Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Thailand Biomarkers Market, By Product
- Consumables
- Services
- Software
- Thailand Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Thailand Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Thailand Biomarkers Market, By Type
- Asia Pacific Biomarkers Market, By Type
- Latin America
- Latin America Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Latin America Biomarkers Market, By Product
- Consumables
- Services
- Software
- Latin America Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Latin America Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Brazil
- Brazil Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Brazil Biomarkers Market, By Product
- Consumables
- Services
- Software
- Brazil Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Brazil Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Brazil Biomarkers Market, By Type
- Mexico
- Mexico Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Mexico Biomarkers Market, By Product
- Consumables
- Services
- Software
- Mexico Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Mexico Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Mexico Biomarkers Market, By Type
- Argentina
- Argentina Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Argentina Biomarkers Market, By Product
- Consumables
- Services
- Software
- Argentina Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Argentina Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Argentina Biomarkers Market, By Type
- Latin America Biomarkers Market, By Type
- Middle East & Africa
- Middle East & Africa Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Middle East & Africa Biomarkers Market, By Product
- Consumables
- Services
- Software
- Middle East & Africa Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Middle East & Africa Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- South Africa
- South Africa Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- South Africa Biomarkers Market, By Product
- Consumables
- Services
- Software
- South Africa Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- South Africa Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- South Africa Biomarkers Market, By Type
- Saudi Arabia
- Saudi Arabia Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Saudi Arabia Biomarkers Market, By Product
- Consumables
- Services
- Software
- Saudi Arabia Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Saudi Arabia Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Saudi Arabia Biomarkers Market, By Type
- UAE
- UAE Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- UAE Biomarkers Market, By Product
- Consumables
- Services
- Software
- UAE Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- UAE Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- UAE Biomarkers Market, By Type
- Kuwait
- Kuwait Biomarkers Market, By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Kuwait Biomarkers Market, By Product
- Consumables
- Services
- Software
- Kuwait Biomarkers Market, By Application
- Diagnostics
- Drug discovery & development
- Personalized Medicine
- Disease Risk Assessment
- Others
- Kuwait Biomarkers Market, By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cancer
- Kuwait Biomarkers Market, By Type
- Middle East & Africa Biomarkers Market, By Type
- North America
Biomarkers Market Dynamics
Driver: Increasing Funding for Biomarkers
Research aids in developing effective therapies for the treatment of various diseases, such as cancer, cardiovascular diseases, immunological diseases, and neurological diseases. It also helps in understanding how a particular disease can be prevented and diagnosed initially. It has been observed that there has been an increase in public and private investments in the biomarkers market. For instance, in 2018, the Alzheimer’s Drug Discovery Foundation launched a biomarker development program, primarily aimed at developing biomarkers that can easily diagnose Alzheimer’s disease & related dementias, demonstrating targets for novel therapies, and monitoring disease progression. In January 2020, Johnson & Johnson Services, Inc. received a grant from FARE (Food Allergy Research & Education) worth USD 250,000 to propel the research on food allergy biomarkers. This grant is anticipated to help in the potential discovery and validation of allergy biomarkers for developing therapies. This can potentially improve the quality of life for 32 million people suffering from food allergies.
Driver: Increasing Prevalence of Chronic Diseases
A rise in the prevalence of fatal diseases has been observed over the past few years, which includes cancer, diabetes, cardiovascular disorders, and other chronic diseases. One of the major factors leading to an increase in their prevalence is lifestyle changes. According to the American Cancer Society, in 2022, an estimated 1.9 million new cancer cases are expected to be registered in the U.S., accounting for 609,360 deaths. Breast and lung cancers were observed as the most common ones worldwide. According to WHO, in 2022, an estimated 236,740 new cases of lung cancer are expected to be reported in the U.S., accounting for 130,180 deaths. In April 2021, Amgen announced the launch of the Biomarker Assist program to improve patients’ access to biomarker testing for non-small cell lung cancer. These programs are anticipated to increase the acceptance rate of novel biomarker tests, thereby enabling correct and on-time treatment. According to a report published in 2020 by the Institute for Health Metrics and Evaluation, cardiovascular diseases cause about 18.9 million deaths annually.
Restraint: Lack of Reimbursement Policies for Biomarkers Treatment
The process for reimbursement of molecular biomarker medical tests is complicated and differs across countries. Lack of adequate reimbursement for biomarkers tests, especially cancer biomarkers testing, is one of the major factors restraining the market. This becomes a primary hurdle in the adoption of new biomarker tests for the treatment of fatal diseases, such as cancer and cardiovascular diseases. This may further impact players of biomarker tests. Centers for Medicare & Medicaid Services identified around 65 tests under the reimbursement policy. Any test apart from these may not be reimbursed, which creates a challenge for market growth. The information that is generated by biomarkers is not being recognized, which is leading to reimbursement issues. Further issues arise when the tests are eligible for reimbursement, but integration of multigene biomarkers is not possible. In addition, the requests made for reimbursement can take years to get issued. Furthermore, the existing reimbursement system presents other challenges such as the development of personalized medicine and adoption of biomarker-based tests.
What Does This Report Include?
This section will provide insights into the contents included in this biomarkers market report and help gain clarity on the structure of the report to assist readers in navigating smoothly.
Biomarkers market qualitative analysis
-
Industry overview
-
Industry trends
-
Market drivers and restraints
-
Market size
-
Growth prospects
-
Porter’s analysis
-
PESTEL analysis
-
Key market opportunities prioritized
-
Competitive landscape
-
Company overview
-
Financial performance
-
Product benchmarking
-
Latest strategic developments
-
Biomarkers market quantitative analysis
-
Market size, estimates, and forecast from 2018 to 2030
-
Market estimates and forecast for product segments up to 2030
-
Regional market size and forecast for product segments up to 2030
-
Market estimates and forecast for application segments up to 2030
-
Regional market size and forecast for application segments up to 2030
-
Company financial performance
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationResearch Methodology
A three-pronged approach was followed for deducing the biomarkers market estimates and forecasts. The process has three steps: information procurement, analysis, and validation. The whole process is cyclical, and steps repeat until the estimates are validated. The three steps are explained in detail below:
Information procurement: Information procurement is one of the most extensive and important stages in our research process, and quality data is critical for accurate analysis. We followed a multi-channel data collection process for biomarkers market to gather the most reliable and current information possible.
- We buy access to paid databases such as Hoover’s and Factiva for company financials, industry information, white papers, industry journals, SME journals, and more.
- We tap into Grand View’s proprietary database of data points and insights from active and archived monitoring and reporting.
- We conduct primary research with industry experts through questionnaires and one-on-one phone interviews.
- We pull from reliable secondary sources such as white papers and government statistics, published by organizations like WHO, NGOs, World Bank, etc., Key Opinion Leaders (KoL) publications, company filings, investor documents, and more.
- We purchase and review investor analyst reports, broker reports, academic commentary, government quotes, and wealth management publications for insightful third-party perspectives.
Analysis: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilized different methods of biomarkers market data depending on the type of information we’re trying to uncover in our research.
-
Market Research Efforts: Bottom-up Approach for estimating and forecasting demand size and opportunity, top-down Approach for new product forecasting and penetration, and combined approach of both Bottom-up and Top-down for full coverage analysis.
-
Value-Chain-Based Sizing & Forecasting: Supply-side estimates for understanding potential revenue through competitive benchmarking, forecasting, and penetration modeling.
-
Demand-side estimates for identifying parent and ancillary markets, segment modeling, and heuristic forecasting.
-
Qualitative Functional Deployment (QFD) Modelling for market share assessment.
Market formulation and validation: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilize different methods of data analysis depending on the type of information we’re trying to uncover in our research.
-
Market Formulation: This step involves the finalization of market numbers. This step on an internal level is designed to manage outputs from the Data Analysis step.
-
Data Normalization: The final market estimates and forecasts are then aligned and sent to industry experts, in-panel quality control managers for validation.
-
This step also entails the finalization of the report scope and data representation pattern.
-
Validation: The process entails multiple levels of validation. All these steps run in parallel, and the study is forwarded for publishing only if all three levels render validated results.
Biomarkers Market Categorization:
The biomarkers market was categorized into four segments, namely type (Safety, Efficacy, Validation), application (Diagnostics, Drug discovery & development, Personalized Medicine), disease (Cancer, Cardiovascular Diseases, Neurological Diseases, Immunological Diseases), and regions (North America, Europe, Asia Pacific, Latin America, Middle East & Africa).
Segment Market Methodology:
The biomarkers market was segmented into type, application, disease, and regions. The demand at a segment level was deduced using a funnel method. Concepts like the TAM, SAM, SOM, etc., were put into practice to understand the demand. We at GVR deploy three methods to deduce market estimates and determine forecasts. These methods are explained below:
Market research approaches: Bottom-up
-
Demand estimation of each product across countries/regions summed up to from the total market.
-
Variable analysis for demand forecast.
-
Demand estimation via analyzing paid database, and company financials either via annual reports or paid database.
-
Primary interviews for data revalidation and insight collection.
Market research approaches: Top-down
-
Used extensively for new product forecasting or analyzing penetration levels.
-
Tool used invoice product flow and penetration models Use of regression multi-variant analysis for forecasting Involves extensive use of paid and public databases.
-
Primary interviews and vendor-based primary research for variable impact analysis.
Market research approaches: Combined
- This is the most common method. We apply concepts from both the top-down and bottom-up approaches to arrive at a viable conclusion.
Regional Market Methodology:
The biomarkers market was analyzed at a regional level. The global was divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, keeping in focus variables like consumption patterns, export-import regulations, consumer expectations, etc. These regions were further divided into twenty-three countries, namely, the U.S.; Canada; the UK.; Germany; France; Italy; Spain; Denmark; Sweden; Norway; India; China; Japan; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; UAE; South Africa; Kuwait.
All three above-mentioned market research methodologies were applied to arrive at regional-level conclusions. The regions were then summed up to form the global market.
biomarkers market companies & financials:
The biomarkers market was analyzed via companies operating in the sector. Analyzing these companies and cross-referencing them to the demand equation helped us validate our assumptions and conclusions. Key market players analyzed include:
-
F. Hoffmann-La Roche Ltd., F. Hoffmann-La Roche Ltd. is a healthcare company with business operations in the following divisions—pharmaceuticals and diagnostics. It was incorporated in 1896 and is headquartered in Switzerland. Roche Diagnostics comprises Roche Professional Diagnostics (RPD), Roche Molecular Diagnostics (RMD), Roche Tissue Diagnostics, and Roche Diabetes Care. The product portfolio of Roche Diagnostics includes blood glucose meters, point-of-care testing devices, high-throughput analyzers for commercial & hospital diagnostic laboratories, and reagents & instruments for life sciences research. RMD, a segment of Roche Diagnostics, is engaged in the development, manufacture, and distribution of tests based on PCR technology & medical diagnostic products, platforms, and services. RMD’s automated platforms and diagnostic tests focus on areas, such as virology, genomics & oncology, blood screening tests, Human Papillomavirus (HPV) & Neisseria gonorrhea/Chlamydia trachomatis, and microbiology.
-
Abbott, Abbott has four core business segments—pharmaceuticals, devices, nutrition, and diagnostics. It was incorporated in 1888 and is headquartered in the U.S. The company has a presence in more than 150 countries with research, manufacturing, sales, and distribution facilities. Abbott’s pharmaceuticals segment offers generic medicines for pain, fever, migraine, inflammation, pancreatic disorders, & hypertension; cardiovascular & metabolic products; and vaccines. The diagnostic segment offers reagents and tests for various applications. The company’s diagnostics portfolio includes technologies such as polymerase chain reaction, fluorescence in situ hybridization, and DNA & RNA sequencing. Abbott’s immunoassays and clinical chemistry assays are used in the diagnosis of cancer, infectious diseases, cardiovascular diseases, fertility, and therapeutic drug monitoring. It employs over 109,000 people globally.
-
Epigenomics AG, Epigenomics AG is a molecular diagnostic company headquartered in Berlin, Germany. It was established in 1998. Epigenomics AG is engaged in the development and commercialization of diagnostic tests for cancer screening, diagnosis, and patient management. Some of the key products in the company’s portfolio are Epi proColon and Epi proLung—diagnostic tests for colorectal cancer and lung cancer, respectively. In addition, the company has identified additional DNA methylation biomarkers via R&D for different cancers, including prostate, breast, bladder, and ovarian cancers. The company employs 33 people.
-
General Electric, GE Healthcare Company operates as a subsidiary of General Electric (GE) Company. It was established in 1892 and is headquartered in Chicago, U.S. The company offers medical technologies and solutions to healthcare entities worldwide. The company specializes in cardiology, orthopedics, and ambulatory surgery centers. It offers products for bone health, EP recording, clinical parameters, accessories, computed tomography, advanced visualization, diagnostic cardiology, hemodynamic recording, metabolic health, patient monitoring, healthcare IT, mammography, interventional image-guided systems, life sciences, perioperative care, radiography, fluoroscopy, magnetic resonance imaging, molecular imaging, nuclear imaging agents, perinatal care, respiratory care, surgical imaging, and ultrasound. The company was formerly known as GE Medical Systems and changed its name to GE Healthcare Limited in October 2003. The company had an employee strength of approximately 168,000 as of 2021.
-
Johnson & Johnson Services, Inc., Johnson & Johnson Services, Inc. is dedicated to developing products related to human health and well-being. It was incorporated in 1886 and is headquartered in the U.S. The company has more than 250 operating units in more than 60 countries. It operates via three business units: consumer healthcare, pharmaceuticals, and medical devices. The consumer healthcare business unit has seven key franchises—nutritional products, skin & hair care, wound care, topical and over-the-counter drugs, oral health care, women’s health, and baby care. Janssen Pharmaceuticals, Inc.—a subsidiary of the company—is involved in the production of pharmaceutical and consumer products. The pharmaceuticals division consists of an array of drugs for neurological conditions, cancer, pain, and autoimmune diseases. As of December 2021, the company employs over 141,700 people globally.
-
Thermo Fisher Scientific, Inc., Thermo Fisher Scientific, Inc. manufactures and supplies scientific equipment, analytical instruments, and other laboratory equipment worldwide. It was incorporated in 1956 and is headquartered in the U.S. The company’s operations are divided into the following sections: pharmaceutical & biotechnology, industrial & applied research, diagnostics & healthcare, and academic & government. It provides chromatographs, Erlenmeyer flasks, gene sequencers & spectrometers, clinical analytical tools, and diagnostic testing products. The company serves more than 400,000 customers globally through its various brands, including Applied Biosystem, Fisher Scientific, Thermo Scientific, Unity Lab Services, and Invitrogen. Its major products include molecular biology reagents, instruments, antibodies, protein biology reagents, and cell imaging & analysis instruments. As of December 2021, the company has an employee strength of 130,000.
-
Bio-Rad Laboratories, Inc., Bio-Rad Laboratories, Inc. manufactures systems and products used in separating complex biological and chemical materials as well as analyzing, identifying, and purifying their respective components. It was incorporated in 1952 and is headquartered in the U.S. The company works in two segments: clinical diagnostics and life sciences. In the clinical diagnostics segment, it designs, manufactures, supports, and sells informatics systems, test systems, quality controls, and test kits. These products serve clinical laboratories in the global market. Similarly, the life sciences segment is employed in the creation and discovery of tools required in biological processes. The company manufactures reagents, apparatus, and laboratory instruments used in the process of biopharmaceutical production, research techniques, & food testing regimes. The company’s products have main applications in hospitals, research institutions, laboratories, biotechnology companies, and pharmaceutical firms. The company has direct distribution channels in approximately 35 countries. The company has an employee strength of approximately 7,900 (as of December 2021).
-
Siemens Healthineers AG, Siemens Healthineers AG is a provider of healthcare solutions such as diagnostics (in vitro & in vivo), imaging & therapy systems, clinical products, audiology solutions, and healthcare IT solutions. The company was founded as a separate entity in 2015. It also offers medical accessories, OEM component & medical electronics solutions, refurbished systems for medical imaging & therapy, technical, maintenance, and professional & consulting services. Its products and solutions are used in various areas, including therapeutic drug monitoring cardiology, organ transplant, urology, infectious disease, diabetes, women's health, surgery, oncology, neurology, and growth disorders. The company has around 18,500 patents worldwide in over 70 countries. It employs over 66,000 employees globally.
-
Qiagen, QIAGEN is a holding company that caters to the biotechnology industry. It was incorporated in 1984 and is headquartered in Germany. The company provides samples and assay technologies for academic research, applied testing, molecular diagnostics, and pharmaceutical research. It markets over 500 products, including consumable kits and automation systems. Its products for molecular diagnostics cover relevant areas of healthcare, ranging from early diagnosis of diseases to establishment of diagnosis to diagnostic testing for determination of suitable treatment to point-of-need testing. The company’s customers include healthcare providers, pharmaceutical & biotechnology companies, government or industrial customers, and researchers. The company employs 5,200 employees globally.
Value chain-based sizing & forecasting
Supply Side Estimates
-
Company revenue estimation via referring to annual reports, investor presentations, and Hoover’s.
-
Segment revenue determination via variable analysis and penetration modeling.
-
Competitive benchmarking to identify market leaders and their collective revenue shares.
-
Forecasting via analyzing commercialization rates, pipelines, market initiatives, distribution networks, etc.
Demand side estimates
-
Identifying parent markets and ancillary markets
-
Segment penetration analysis to obtain pertinent
-
revenue/volume
-
Heuristic forecasting with the help of subject matter experts
-
Forecasting via variable analysis
Biomarkers Market Report Objectives:
-
Understanding market dynamics (in terms of drivers, restraints, & opportunities) in the countries.
-
Understanding trends & variables in the individual countries & their impact on growth and using analytical tools to provide high-level insights into the market dynamics and the associated growth pattern.
-
Understanding market estimates and forecasts (with the base year as 2022, historic information from 2018 to 2021, and forecast from 2023 to 2030). Regional estimates & forecasts for each category are available and are summed up to form the global market estimates.
Biomarkers Market Report Assumptions:
-
The report provides market value for the base year 2022 and a yearly forecast till 2030 in terms of revenue/volume or both. The market for each of the segment outlooks has been provided on region & country basis for the above-mentioned forecast period.
-
The key industry dynamics, major technological trends, and application markets are evaluated to understand their impact on the demand for the forecast period. The growth rates were estimated using correlation, regression, and time-series analysis.
-
We have used the bottom-up approach for market sizing, analyzing key regional markets, dynamics, & trends for various products and end-users. The total market has been estimated by integrating the country markets.
-
All market estimates and forecasts have been validated through primary interviews with the key industry participants.
-
Inflation has not been accounted for to estimate and forecast the market.
-
Numbers may not add up due to rounding off.
-
Europe consists of EU-8, Central & Eastern Europe, along with the Commonwealth of Independent States (CIS).
-
Asia Pacific includes South Asia, East Asia, Southeast Asia, and Oceania (Australia & New Zealand).
-
Latin America includes Central American countries and the South American continent
-
Middle East includes Western Asia (as assigned by the UN Statistics Division) and the African continent.
Primary Research
GVR strives to procure the latest and unique information for reports directly from industry experts, which gives it a competitive edge. Quality is of utmost importance to us, therefore every year we focus on increasing our experts’ panel. Primary interviews are one of the critical steps in identifying recent market trends and scenarios. This process enables us to justify and validate our market estimates and forecasts to our clients. With more than 8,000 reports in our database, we have connected with some key opinion leaders across various domains, including healthcare, technology, consumer goods, and the chemical sector. Our process starts with identifying the right platform for a particular type of report, i.e., emails, LinkedIn, seminars, or telephonic conversation, as every report is unique and requires a differentiated approach.
We send out questionnaires to different experts from various regions/ countries, which is dependent on the following factors:
-
Report/Market scope: If the market study is global, we send questionnaires to industry experts across various regions, including North America, Europe, Asia Pacific, Latin America, and MEA.
-
Market Penetration: If the market is driven by technological advancements, population density, disease prevalence, or other factors, we identify experts and send out questionnaires based on region or country dominance.
The time to start receiving responses from industry experts varies based on how niche or well-penetrated the market is. Our reports include a detailed chapter on the KoL opinion section, which helps our clients understand the perspective of experts already in the market space.
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationShare this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





