- Home
- »
- Medical Devices
- »
-
Drug Device Combination Products Market Size Report, 2030GVR Report cover
![Drug Device Combination Products Market Size, Share & Trends Report]()
Drug Device Combination Products Market Size, Share & Trends Analysis Report By Product (Transdermal Patches, Infusion Pumps, Inhalers, Drug Eluting Stents), By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-1-68038-260-0
- Number of Pages: 105
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Market Size & Trends
The global drug device combination products market size was valued at USD 127.8 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 8.9% from 2023 to 2030. The market is primarily driven by growing patient and physician preference for minimally invasive procedures and consistent-dosing treatment alternatives. The pandemic positively impacted this market space as the use of certain combination products was increased during the different waves of COVID-19. In November 2020, the U.S. Food and Drug Administration has issued an emergency use authorization (EUA) for baricitinib in combination with remdesivir in hospitalized adults and pediatric patients two years of age and older who require supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation for suspected or laboratory confirmed COVID-19 (ECMO).
Article 117 of the Medical Devices Regulation (MDR) requires manufacturers to obtain a Notified Body Opinion before placing drug-device combination goods on the market as an integral device and marketing them as a "medicinal product" (NBOp). The notified body then certifies that the device complies with the relevant General Safety and Performance Requirements (GSPR) and sends the manufacturer an NBOp Report to include in the Market Authorization Application (MAA). Autoinjectors, inhalers, pre-filled nebulizers, pre-filled pens, prefilled syringes, and transdermal patches are examples of drug-device combination items that require NBOp.
The subsequent increase in the adoption of these products can be credited to associated benefits such as reduced pain levels, better patient outcomes, reduced hospital stay, and overall healthcare cost-efficiency. Other advantages include synergistic effects facilitating multi-target treatment, improved tolerance levels, simplification of dosage regime, and improved symptomatic and pharmacokinetic profiles. These benefits are expected to propel the demand for these systems and present the market with numerous growth opportunities.
In addition, frequent intervention by government health organizations to ensure high patient safety is predominantly driving the market. To address the challenges associated with drug-device combination products as well as facilitate proper and consistent regulation, the U.S. FDA has established the Office of Combination Products (OCP). Moreover, unprecedented growth in the development of clinical drugs and devices by large pharmaceutical companies is believed to meet the demand for advanced medication delivery technologies. This has resulted in extensive product pipelines and maximized commercial returns on already established products, thereby serving as a significant driving factor for the industry.
Product Insights
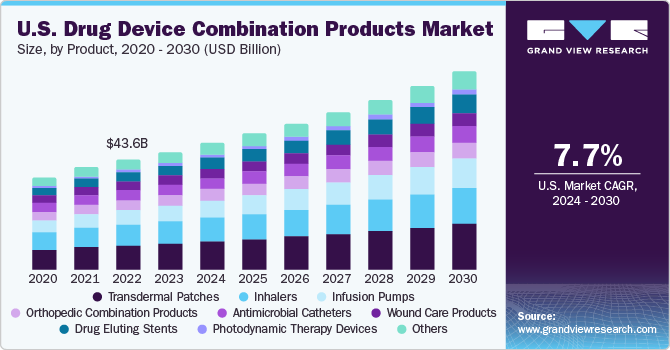
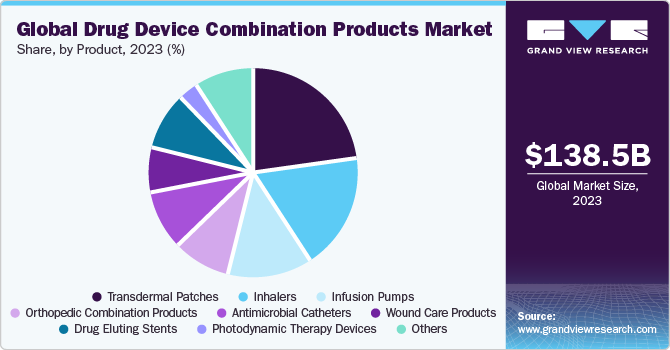
Transdermal patches emerged as the largest segment in 2022 with over 33.9% share. This can be attributed to the increasing demand for self-administration of drugs in cases of diseases requiring long-term treatment. For instance, in diabetes, insulin is needed to be frequently administered intravenously into the patient’s body. This repeated use of injections to deliver insulin causes pain and increases the risk of infection transmission, thus leading to heightened growth prospects for alternative drug delivery modes.
Advantages associated with the adoption of transdermal patches include better patient compliance and reduced risk of needlestick injuries and infections, which further accelerates the demand for these patches. Moreover, rising patient preference for pain-free drug delivery has led to a significant surge in the adoption of adhesive skin patches to administer drugs. This is expected to present the market with tremendous growth potential over the forecast period.
The inhalers segment is anticipated to exhibit a lucrative growth rate over the forecast period owing to the rising prevalence of chronic diseases such as tuberculosis, diabetes mellitus, and chronic obstructive pulmonary disease, which require more rigorous treatment. Technological advancements such as particle engineering, agglomerated vesicle technology, incorporation of MDI technology, and supercritical fluid technology are expected to boost segment growth.
Regional Insights
North America held the dominant share of over 40.0% in 2022. This can be attributed to the extensive new product development activities conducted by prominent players across this region. The rising chronic disease burden in this region is believed to be one of the key potential growth factors responsible for the high clinical urgency to incorporate these products. This is believed to contribute to the growth of the North American market.
Asia Pacific is anticipated to grow at a lucrative rate over the forecast period. The sizable growth is attributed to the increasing healthcare spending. Rising physician awareness levels pertaining to the benefits of these products, including low dosage requirement, timed release of medication, direct mitigation of device-centered infection using anti-infective medication in combination, and reduced systemic exposure, also contribute to the regional market growth. The market is driven by high R&D investments by global market players as well as increasing commercialization of these systems developed by key players.
Moreover, the urgent need for the development of new systems and replacement and upgrading of medical infrastructure is anticipated to present the APAC market with lucrative growth opportunities over the forecast period. Stringent regulatory policies issued by healthcare organizations to ensure high safety standards are further expected to widen the scope for growth across this region.
Key Companies & Market Share Insights
The key players are engaged in extensive expansion strategies, new product development activities, and mergers and acquisitions, which have significantly contributed to their growth. High operational costs, stringent regulatory framework, and capital requirements keep entry barriers at a higher level, owing to which, the threat of new entrants is expected to be low. Some prominent players in the global drug device combination products market include:
-
Abbott
-
Terumo Corporation
-
Stryker Corporation
-
Mylan N.V.
-
Medtronic
-
Allergan plc
-
Boston Scientific Corporation
-
Novartis AG
-
C.R. Bard
-
Teleflex Incorporated
-
W. L. Gore & Associates, Inc.
Drug Device Combination Products Market Report scope
Report Attribute
Details
Market size value in 2023
USD 138.48 billion
Revenue forecast in 2030
USD 251.87 billion
Growth rate
CAGR of 8.9% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
June 2023
Quantitative units
Revenue in USD billion, and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S., Canada, U.K., Germany, France, Italy, Spain, Denmark, Norway, Sweden; China, Japan, India, Australia, South Korea, Thailand; Mexico, Brazil, Argentina, South Africa, Saudi Arabia, UAE, Kuwait
Key companies profiled
Abbott; Terumo Corporation; Stryker Corporation; Mylan N.V.; Medtronic; Allergan plc; Boston Scientific Corporation; Novartis AG; C.R. Bard; Teleflex Incorporated; W. L. Gore & Associates, Inc.
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional, and segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Drug Device Combination Products Market Report Segmentation
This report forecasts revenue growth at global, regional & country levels and provides an analysis on the latest trends and opportunities in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global drug device combination products market report on the basis of product, and region:
-
Product Outlook (Revenue, USD Billion, 2018 - 2030)
-
Infusion Pumps
-
Volumetric
-
Disposables
-
Syringes
-
Ambulatory
-
Implantable
-
Insulin
-
-
Orthopedic Combination Products
-
Bone Graft Implants
-
Antibiotic Bone Cement
-
-
Photodynamic Therapy Devices
-
Transdermal Patches
-
Drug Eluting Stents
-
Coronary Stents
-
Peripheral Vascular Stents
-
-
Wound Care Products
-
Inhalers
-
Dry Powder
-
Nebulizers
-
Metered Dose
-
-
Antimicrobial Catheters
-
Urological
-
Cardiovascular
-
Others
-
-
Others
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
U.K.
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global drug device combination products market size was estimated at USD 127.8 billion in 2022 and is expected to reach USD 138.48 billion in 2023.
b. The global drug device combination products market is expected to grow at a compound annual growth rate of 8.9% from 2023 to 2030 to reach USD 251.78 billion by 2030.
b. North America dominated the drug device combination products market with a share of 40.51% in 2022. This is attributable to extensive new product development activities conducted by prominent players across this region.
b. Some key players operating in the drug device combination products market include Abbott; Terumo Corporation; Stryker Corporation; Mylan N.V.; Medtronic; Allergan plc; Boston Scientific Corporation; Novartis AG; C.R. Bard; Teleflex Incorporated; and W. L. Gore & Associates, Inc.
b. Key factors that are driving the drug device combination products market growth include growing patient and physician preference for minimally invasive procedures and consistent-dosing treatment alternatives.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





