- Home
- »
- Biotechnology
- »
-
Gene Therapy Market Size, Share & Growth Report, 2030GVR Report cover
![Gene Therapy Market Size, Share & Trend Report]()
Gene Therapy Market Size, Share & Trend Analysis Report By Indication (Acute Lymphoblastic Leukemia, Large B-cell Lymphoma), By Vector Type (Lentivirus), By Route of Administration, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-2-68038-179-5
- Number of Pages: 162
- Format: Electronic (PDF)
- Historical Range: 2018 - 2022
- Industry: Healthcare
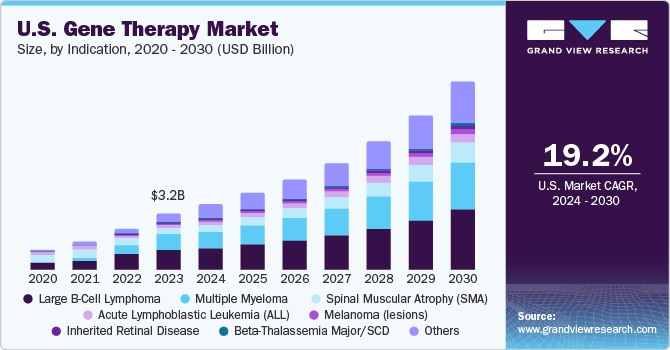
Gene Therapy Market Size & Trends
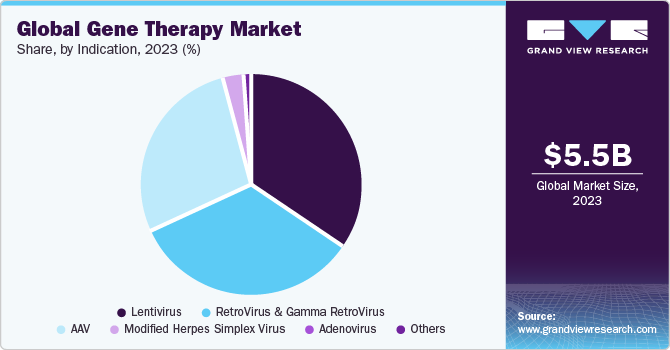
The global gene therapy market size was estimated at USD 8.67 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 19.5% from 2024 to 2030. The growth of the market is attributed to many factors such as expanding area of advanced therapies along with gene delivery technologies and progressive competition among key players focused on commercialization of their therapies. The biotechnology companies are investing in acquisitions, mergers/collaborations, and deals as a key strategy to increase in-house expertise and strengthen the product pipelines.
The COVID-19 outbreak has negatively impacted the market growth. This sector has experienced severe disruption due to COVID-19, which has historically presented significant challenges in the supply of materials, manufacturing, and logistics operations. For instance, companies had lengthy delivery times for specific components and later discovered that it was short on clinical trial supplies when a partner contract manufacturing company was compelled to shut down.
The robust pipeline is expected to boost the market growth over the forecast period. Researchers are working to make gene therapy available at clinics. Various universities and institutes exhibit a broad portfolio of products in the pipeline which is expected to boost revenue generation over the forecast period. Clinical trials for gene therapy increased significantly from 2017 to 2018, after the FDA approved first gene therapy. According to the American Society of Gene & Cell Therapy (ASGCT), around 1,986 products, including CAR T-cell therapies and other genetically modified cell therapies, are currently under development.
Moreover, improving regulatory support creates growth opportunities for the market over the forecast period. Several positive changes have been made by many international regulatory organizations to promote therapies. Support for CAR-T technology from the FDA is one of the examples. In phase II and III studies, in particular, regulators are allowing flexibility in the usual hierarchy of how clinical trials are conducted. Moreover, FDA expects 10 to 20 new therapies to be approved annually by 2025.
Furthermore, an increase in funding and investments in this sector is expected to provide lucrative growth opportunities to market players. Several biopharma companies are investing in this sector for novel product launches. For instance, in January 2022, Ori Biotech raised more than USD 100.0 million in Series B funding to introduce a novel cell & gene therapy developing platform. This funding allowed for a rapid transition from pre-commercialization to market launch.
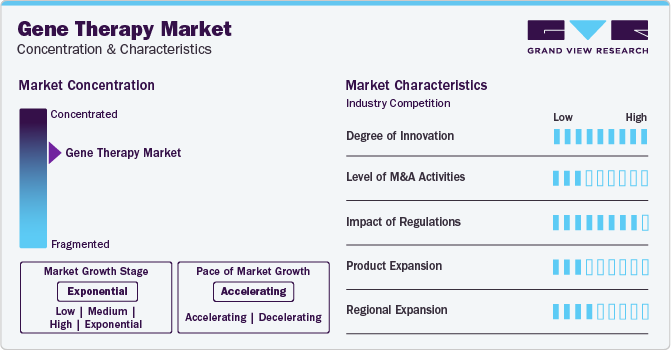
Market Concentration & Characteristics
Market growth for gene therapy market is exponential and market growth is accelerating. The increasing number of approvals of gene therapies within North America and Europe are the major reasons for market growth. Furthermore, strong pipeline of numerous companies is anticipated to bring new therapies in the market.
Some of the major strategies opted by the companies within gene therapy market include increased spending on R&D and new product launches. For instance, in December 2023, the U.S. FDA approved a gene therapy for treating sickle cell disease. This is the first gene therapy that is based on CRISPR gene editing. Such new launches is expected to fuel the market growth over the forecast period.
The gene therapy market has very high impact of regulations. Regional regulatory bodies such as the U.S. Food and Drug Administration, European Medicines Agency, and more are responsible for all the approvals of therapies. Lengthy approval processes and clinical trials limit the entry of new players within the market.
Currently, North America and Europe are the regions which have the most approved gene therapies as of January 2024. However, in countries such as India, South Korea, Japan, large number of clinical trials are being undertaken for various conditions. For instance, in July 2023, a team of researchers at the Narayana Nethralaya announced that they will be starting a clinical trial for a gene therapy for ocular diseases.
Vector Insights
The AAV segment shows a significant revenue contribution of 22% in 2023. Several biopharma companies are offering their viral vector platform for the development of AAV-based gene therapy product. For instance, in September 2016, Lonza signed an exclusive agreement with Massachusetts Eye and Ear to support its novel Anc-AAV gene therapy platform for development and commercialization of next-generation gene therapies based on their AAV platform. Similarly, RegenxBio had made an agreement with companies AveXis & Biogen in March 2014 and May 2016, respectively, which would allow both companies to use RegenxBio’s AAV vector platform for development of gene therapy molecules. Furthermore, in May 2021, Biogen Inc. and Capsigen Inc. entered into a strategic research partnership to engineer novel AAV capsids that have the possibility to deliver transformative gene therapies, which can address the fundamental genetic causes of numerous neuromuscular and CNS disorders. In July 2021, the U.S. Department of Commerce’s National Institute of Standards and Technology (NIST), National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL), and United States Pharmacopeia (USP) announced a collaboration to evaluate analytical methods and develop standards for AAV. As part of this partnership, NIST and USP will be conducting an interlaboratory study in which several laboratories will measure these serious quality attributes, and their results will be linked and examined. This collaboration will support the development of new promising gene therapies that will significantly advance people’s lives.
Indication Insights
The spinal muscular atrophy (SMA) segment dominated the market in 2023. Although SMA is a rare disorder, it is one of the most common fatal inherited diseases of infancy. The development of Zolgensma (AVXS-101), has proven its effectiveness in treating SMA and altering the phenotype of the illness. The FDA approved Novartis' Zolgensma approval in May 2019, which is aimed at treating the underlying cause of SMA. As of now, Zolgensma is the only gene treatment in this field to have been approved. The approval of this gene therapy is evidence of the growing use of therapies to treat serious hereditary illnesses like SMA.
The Beta-Thalassemia Major/SCD segment is anticipated to register the fastest CAGR over the forecast period. Gene therapy for SCD and β-thalassemia is based on transplantation of gene-modified hematopoietic stem cells. Clinical and preclinical studies have shown the efficacy and safety of this therapeutic modality. However, several other factors, such as suboptimal gene expression levels & gene transfer efficiency, limited stem-cell dose and quality, and toxicity of myeloablative regimens are still hampering its efficacy. Despite these challenges, in June 2019, bluebird Bio’s Zynteglo (formerly LentiGlobin) received conditional approval in Europe for the treatment of β-thalassemia and is expected to receive U.S. FDA approval in August 2022. Moreover, the product has already received Orphan Drug status by the U.S. FDA for treatment of patients with sickle cell disease (SCD). Furthermore, in April 2021, Vertex Pharmaceuticals and CRISPR Therapeutics amended partnership for the development, production, and commercialization of CTX001 in sickle beta thalassemia and cell disease. These achievements in this segment are anticipated to significantly boost the adoption of the product in this segment.
Route of Administration Insights
The intravenous segment dominated the global gene therapy market in 2023. Large number of approved products along with strong pipeline for IV candidates is the major reason for the segment dominance. The segment is also expected to emerge as the most lucrative over the forecast period.
Regional Insights
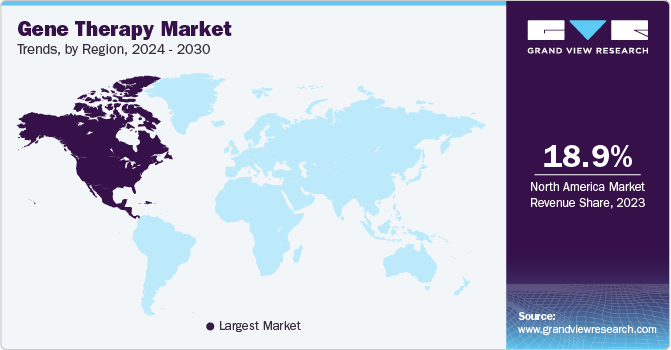
North America dominated the market in 2023 with the largest revenue share of 65.2% in 2023. This region is expected to become the largest routine manufacturer of gene therapy in terms of the number of approvals and revenue generated during the forecast period. Increasing investments in R&D from large and small companies in the development of ideal therapy drugs are anticipated to further boost the market.
Furthermore, the increasing number of investments by the governments and the growing prevalence of targeted diseases are the factors fueling the market. According to the Spinal Muscular Atrophy Foundation, in 2020, around 10,000 to 25,000 children and adults in the U.S. were affected by spinal muscular atrophy, making it a fairly common disease among rare diseases.
Europe is estimated to be the fastest-growing regional segment from 2024 to 2030. This is attributed to its large population with unmet medical needs and increasing demand for novel technologies in the treatment of rare but increasingly prevalent diseases. Asia Pacific market for commercial application of genetic therapies is anticipated to witness significant growth in the forecast period, which can be attributed to the easy availability of resources, local presence of major companies, and increased investment, by the governments.
UK Gene Therapy Market
The UK gene therapy market is anticipated to witness accelerated growth over the forecast period, due to increased investments by various big companies and governments, including the NHS & research laboratories. For instance, in March 2022, the UK government invested USD 326.45 million to accelerate healthcare research and manufacturing. Under this investment, additional $80 million of the fund will help companies at the forefront of invention with their commercial-scale manufacturing investments in areas like gene and cell therapies, as well as improved diagnostic technologies, among others. Various mergers & partnerships between manufacturers, universities, and other government bodies are expected to boost the market over the forecast period.
Key Companies & Market Share Insights
Some of the players operating in the market are REGENXBIO, Inc.; Oxford BioMedica plc; Dimension Therapeutics, Inc.; Bristol-Myers Squibb Company; SANOFI. Some of the emerging players operating in the market are Spark Therapeutics, Neurochase, Orchard Therapeutics, Astellas Pharma Inc. Major companies in the highly competitive gene therapy market are aiming for product approvals to strengthen their market position. Also, companies are stepping into strategic collaboration for the purpose of technology sharing, platform sharing, and knowledge sharing to develop effective gene therapies.
Key Gene Therapy Companies:
- REGENXBIO, Inc.
- Oxford BioMedica plc
- Dimension Therapeutics, Inc.
- Bristol-Myers Squibb Company
- SANOFI
- Applied Genetic Technologies Corporation
- F. Hoffmann-La Roche Ltd
- bluebird Bio, Inc.
- Novartis AG
- Taxus Cardium Pharmaceuticals Group, Inc. (Gene Biotherapeutics)
- UniQure N.V.
- Shire Plc
- Cellectis S.A.
- Sangamo Therapeutics, Inc
- Orchard Therapeutics
- Gilead Lifesciences, Inc.
- BENITEC BIOPHARMA
- Sibiono GeneTech Co., Ltd
- Shanghai Sunway Biotech Co., Ltd.
- Gensight Biologics S.A.
- Transgene
- Calimmune, Inc.
- Epeius Biotechnologies Corp.
- Astellas Pharma Inc.
- American Gene Technologies
- BioMarin Pharmaceuticals, Inc.
Recent Developments
-
In January 2024, Biogen and Ginkgo Bioworks announced that they have completed the gene therapy collaboration involving AAV based vectors. This is expected to fuel the demand for gene therapies in coming years.
-
In December 2023, Swiss Agency for Therapeutic Products granted approval to Libmeldy for treatment of early-onset metachromatic leukodystrophy.
-
In May 2023, Krystal Biotech was granted approval for VYJUVEK gene therapy for Dystrophic Epidermolysis Bullosa treatment
-
In June 2023, the U.S. FDA granted approval to Sarepta for ELEVIDYS gene therapy to treat DMD in children of age 4-5 years
-
In January 2023, Voyager Therapeutics and Neurocrine Biosciences entered into a strategic collaboration for commercialization & development of Voyager’s GBA1 program and other next-generation gene therapies for neurological diseases
-
In January 2023, Spark Therapeutics and Neurochase established a strategic collaboration to develop Neurochase’s unique delivery technology for use with selected gene treatments for rare disorders in the CNS. In this agreement, Neurochase will contribute its extensive knowledge in direct drug delivery technology to Spark’s premier AAV platform.
-
In January 2022, 64x Bio, a U.S.-based biotech company, raised USD 55.0 million in funding to advance its gene therapy manufacturing platform. This initiative was expected to expand the company’s VectorSelect platform.
Gene Therapy Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 10.11 billion
Revenue forecast in 2030
USD 29.51 billion
Growth rate
CAGR of 19.5% from 2024 to 2030
Actual data
2018 - 2023
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million/billion, CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, trends
Segments covered
Vector type, indication, route of administration, region
Regional scope
North America; Europe; Asia Pacific; Rest of World.
Country scope
U.S., Canada, UK, Germany, France, Italy, Spain, Japan, China, South Korea, Australia
Key companies profiled
REGENXBIO, Inc.; Oxford BioMedica plc; Dimension Therapeutics, Inc.; Bristol-Myers Squibb Company; SANOFI; Applied Genetic Technologies Corp; F. Hoffmann-La Roche Ltd.; Bluebird Bio, Inc.; Novartis AG; Taxus Cardium Pharmaceuticals Group, Inc. (Gene Biotherapeutics); UniQure N.V.; Shire Plc; Cellectis S.A.; Sangamo Therapeutics, Inc.; Orchard Therapeutics; Gilead Lifesciences, Inc.; Benitec Biopharma Ltd.; Sibiono GeneTech Co., Ltd.; Shanghai Sunway Biotech Co., Ltd.; Gensight Biologics S.A.; Transgene; Calimmune, Inc.; Epeius Biotechnologies Corp.; Astellas Pharma, Inc.; American Gene Technologies; BioMarin Pharmaceuticals, Inc.
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Global Gene Therapy Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels in addition to provides an analysis of the latest industry trends in each of the subsegments from 2018 to 2030. For this study, Grand View Research has segmented the global gene therapy market report based on the indication, vector type, and region:
-
Indication Outlook (Revenue, USD Million, 2018 - 2030)
-
Acute Lymphoblastic Leukemia (ALL)
-
Inherited Retinal Disease
-
Large B-cell Lymphoma
-
ADA-SCID
-
Melanoma (lesions)
-
Beta-Thalassemia Major/SCD
-
Head & Neck Squamous Cell Carcinoma
-
Peripheral arterial disease
-
Spinal Muscular Atrophy (SMA)
-
Others
-
-
Vector Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Lentivirus
-
AAV
-
RetroVirus & gamma RetroVirus
-
Modified Herpes Simplex Virus
-
Adenovirus
-
Non-viral Plasmid Vector
-
Others
-
-
Route of Administration (Revenue, USD Million; 2018 - 2030)
-
Intravenous
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
-
Asia Pacific
-
Japan
-
China
-
South Korea
-
Australia
-
-
Rest of the world
-
Frequently Asked Questions About This Report
b. The global gene therapy market size was estimated at USD 8.67 billion in 2023 and is expected to reach USD 10.12 billion in 2024.
b. The global gene therapy market is expected to grow at a compound annual growth rate of 19.5% from 2024 to 2030 to reach USD 29.51 billion by 2030.
b. North America dominated the gene therapy market with a share of 65.2% in 2023. This is attributable to rising healthcare awareness coupled with the growing demand for robust therapeutics to treat chronic illness.
b. Some key players operating in the gene therapy market include Novartis AG; Spark Therapeutics LLC; Bluebird Bio; Gilead Sciences Inc.; Celgene Corporation; Shire Plc; Voyager Therapeutics; Dimension Therapeutics; Chiesi Farmaceutici S.p.A; and Celgene Corporation.
b. Key factors that are driving the gene therapy market growth include recent approval of products such as Zolgensma and LentiGlobin which has accelerated investment in clinical trials of pipeline programs.
Table of Contents
Chapter 1. Gene Therapy Market: Methodology and Scope
1.1. Market Segmentation & Scope
1.2. Market Definitions
1.2.1. Information Analysis
1.2.2. Market Application & Data Visualization
1.2.3. Data Validation & Publishing
1.3. Research Assumptions
1.4. Information Procurement
1.4.1. Primary Research
1.5. Information or Data Analysis
1.6. Market Application & Validation
1.7. Market Model
1.8. Global Market: CAGR Calculation
1.9. Objectives
1.9.1. Objective 1
1.9.2. Objective 2
Chapter 2. Gene Therapy Market: Executive Summary
2.1. Market Snapshot
2.2. Segment Snapshot
2.3. Competitive Landscape Snapshot
Chapter 3. Gene Therapy Market: Variables, Trends, & Scope
3.1. Market Lineage Outlook
3.1.1. Parent Market Outlook
3.1.2. Related/Ancillary Market Outlook
3.2. Market Dynamics
3.2.1. Market Driver Analysis
3.2.1.1. Robust Gene Therapy Pipeline
3.2.1.2. Introduction Of Technological Advancements
3.2.1.3. Increasing Investment From Companies And Partnerships
3.2.1.4. Growing Prevalence Of Target Diseases And Increased Demand For Innovative Medicine
3.2.2. Market Restraint Analysis
3.2.2.1. Absence Of Effective Diagnosis Framework
3.2.2.2. High Prices Of Gene Therapy
3.2.3. Market Opportunity Analysis
3.2.3.1. Rising Investment For Adoptive T-Cell Transfer Approaches Of Disease Treatment
3.2.3.2. Facility Expansion For Cell And Gene Therapies
3.2.3.3. Technological Advancements In Manufacturing Vectors
3.3. Industry Analysis Tools
3.3.1. Porter’s Five Forces Analysis
3.3.2. PESTEL Analysis
3.3.3. COVID-19 Impact Analysis
3.4. Pipeline Analysis
3.4.1. Key takeaways from 2023
3.4.2. Gene therapy pipeline: most common targets
3.5. Payment & Pricing Models
3.5.1. Payment models for innovative therapies
Chapter 4. Route of Administration Business Analysis
4.1. Gene Therapy Market: Route of Administration Movement Analysis
4.2. Intravenous
4.2.1. Intravenous Market, 2018 - 2030 (USD Million)
4.3. Others
4.3.1. Others Market, 2018 - 2030 (USD Million)
Chapter 5. Indication Business Analysis
5.1. Gene Therapy Market: Indication Movement Analysis
5.2. Acute Lymphoblastic Leukemia
5.2.1. Acute Lymphoblastic Leukemia Market, 2018 - 2030 (USD Million)
5.3. Inherited Retinal Disease
5.3.1. Inherited Retinal Disease Market, 2018 - 2030 (USD Million)
5.4. Large B-Cell Lymphoma
5.4.1. Large B-Cell Lymphoma Market, 2018 - 2030 (USD Million)
5.5. Adenosine Deaminase (Ada)‐Deficient Severe Combined Immunodeficiency (Scid)
5.5.1. Adenosine Deaminase (Ada)‐Deficient Severe Combined Immunodeficiency (Scid) Market, 2018 - 2030 (USD Million)
5.6. Melanoma (lesions)
5.6.1. Melanoma (lesions) Market, 2018 - 2030 (USD Million)
5.7. Beta-Thalassemia Major/Sickle Cell Disease (Scd)
5.7.1. Beta-Thalassemia Major/Sickle Cell Disease (Scd) Market, 2018 - 2030 (USD Million)
5.8. Head & Neck Squamous Cell Carcinoma
5.8.1. Head & Neck Squamous Cell Carcinoma Market, 2018 - 2030 (USD Million)
5.9. Peripheral arterial disease
5.9.1. Peripheral arterial disease Market, 2018 - 2030 (USD Million)
5.10. Spinal Muscular Atrophy (SMA)
5.10.1. Spinal Muscular Atrophy (SMA) Market, 2018 - 2030 (USD Million)
5.11. Others
5.11.1. Others Market, 2018 - 2030 (USD Million)
Chapter 6. Vector Type Business Analysis
6.1. Gene Therapy Market: Vector Type Movement Analysis
6.2. Lentiviral Vectors
6.2.1. Lentiviral Vectors market, 2018 - 2030 (USD Million)
6.3. Adeno-Associated Viral (Aav) Vectors
6.3.1. Adeno-Associated Viral (Aav) Vectors market, 2018 - 2030 (USD Million)
6.4. Retrovirus Vectors
6.4.1. Retrovirus Vectors market, 2018 - 2030 (USD Million)
6.5. Modified Herpes Simplex Virus
6.5.1. Modified Herpes Simplex Virus market, 2018 - 2030 (USD Million)
6.6. Adenovirus Vectors
6.6.1. Adenovirus Vectors market, 2018 - 2030 (USD Million)
6.7. Non-Viral Plasmid Vector
6.7.1. Non-Viral Plasmid Vector market, 2018 - 2030 (USD Million)
6.8. Others
6.8.1. Others market, 2018 - 2030 (USD Million)
Chapter 7. Regional Business Analysis
7.1. Gene Therapy Market Share By Region, 2023 & 2030
7.2. North America
7.2.1. North America Gene Therapy Market, 2018 - 2030 (USD Million)
7.2.2. U.S.
7.2.2.1. Key Country Dynamics
7.2.2.2. Target disease prevalence
7.2.2.3. Competitive Scenario
7.2.2.4. Regulatory Framework
7.2.2.5. U.S. Gene Therapy Market, 2018 - 2030 (USD Million)
7.2.3. Canada
7.2.3.1. Key Country Dynamics
7.2.3.2. Target disease prevalence
7.2.3.3. Competitive Scenario
7.2.3.4. Regulatory Framework
7.2.3.5. Canada Gene Therapy Market, 2018 - 2030 (USD Million)
7.3. Europe
7.3.1. Europe Gene Therapy Market, 2018 - 2030 (USD Million)
7.3.2. UK
7.3.2.1. Key Country Dynamics
7.3.2.2. Target disease prevalence
7.3.2.3. Competitive Scenario
7.3.2.4. Regulatory Framework
7.3.2.5. UK Gene Therapy Market, 2018 - 2030 (USD Million)
7.3.3. Germany
7.3.3.1. Key Country Dynamics
7.3.3.2. Target disease prevalence
7.3.3.3. Competitive Scenario
7.3.3.4. Regulatory Framework
7.3.3.5. Germany Gene Therapy Market, 2018 - 2030 (USD Million)
7.3.4. France
7.3.4.1. Key Country Dynamics
7.3.4.2. Target disease prevalence
7.3.4.3. Competitive Scenario
7.3.4.4. Regulatory Framework
7.3.4.5. France Gene Therapy Market, 2018 - 2030 (USD Million)
7.3.5. Italy
7.3.5.1. Key Country Dynamics
7.3.5.2. Target disease prevalence
7.3.5.3. Competitive Scenario
7.3.5.4. Regulatory Framework
7.3.5.5. Italy Gene Therapy Market, 2018 - 2030 (USD Million)
7.3.6. Spain
7.3.6.1. Key Country Dynamics
7.3.6.2. Target disease prevalence
7.3.6.3. Competitive Scenario
7.3.6.4. Regulatory Framework
7.3.6.5. Spain Gene Therapy Market, 2018 - 2030 (USD Million)
7.4. Asia Pacific
7.4.1. Asia Pacific Gene Therapy Market, 2018 - 2030 (USD Million)
7.4.2. Japan
7.4.2.1. Key Country Dynamics
7.4.2.2. Target disease prevalence
7.4.2.3. Competitive Scenario
7.4.2.4. Regulatory Framework
7.4.2.5. Japan Gene Therapy Market, 2018 - 2030 (USD Million)
7.4.3. China
7.4.3.1. Key Country Dynamics
7.4.3.2. Target disease prevalence
7.4.3.3. Competitive Scenario
7.4.3.4. Regulatory Framework
7.4.3.5. China Gene Therapy Market, 2018 - 2030 (USD Million)
7.4.4. Australia
7.4.4.1. Key Country Dynamics
7.4.4.2. Target disease prevalence
7.4.4.3. Competitive Scenario
7.4.4.4. Regulatory Framework
7.4.4.5. Australia Gene Therapy Market, 2018 - 2030 (USD Million)
7.5. Rest of the world
7.5.1. Rest of the world Gene Therapy Market, 2018 - 2030 (USD Million)
Chapter 8. Competitive Landscape
8.1. Company Categorization
8.2. Strategy Mapping
8.3. Company Market Position Analysis, 2023
8.4. Company Profiles
8.4.1. REGENXBIO, INC
8.4.1.1. Overview
8.4.1.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.1.3. Product Benchmarking
8.4.1.4. Strategic Initiatives
8.4.2. OXFORD BIOMEDICA PLC
8.4.2.1. Overview
8.4.2.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.2.3. Product Benchmarking
8.4.2.4. Strategic Initiatives
8.4.3. VOYAGER THERAPEUTICS
8.4.3.1. Overview
8.4.3.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.3.3. Product Benchmarking
8.4.3.4. Strategic Initiatives
8.4.4. HUMAN STEM CELLS INSTITUTE
8.4.4.1. Overview
8.4.4.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.4.3. Product Benchmarking
8.4.4.4. Strategic Initiatives
8.4.5. DIMENSION THERAPEUTICS, INC
8.4.5.1. Overview
8.4.5.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.5.3. Product Benchmarking
8.4.5.4. Strategic Initiatives
8.4.6. BRISTOL-MYERS SQUIBB COMPANY
8.4.6.1. Overview
8.4.6.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.6.3. Product Benchmarking
8.4.6.4. Strategic Initiatives
8.4.7. SANOFI
8.4.7.1. Overview
8.4.7.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.7.3. Product Benchmarking
8.4.7.4. Strategic Initiatives
8.4.8. APPLIED GENETIC TECHNOLOGIES CORPORATION
8.4.8.1. Overview
8.4.8.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.8.3. Product Benchmarking
8.4.8.4. Strategic Initiatives
8.4.9. F. HOFFMANN-LA ROCHE LTD
8.4.9.1. Overview
8.4.9.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.9.3. Product Benchmarking
8.4.9.4. Strategic Initiatives
8.4.10. BLUEBIRD BIO, INC
8.4.10.1. Overview
8.4.10.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.10.3. Product Benchmarking
8.4.10.4. Strategic Initiatives
8.4.11. NOVARTIS AG
8.4.11.1. Overview
8.4.11.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.11.3. Product Benchmarking
8.4.11.4. Strategic Initiatives
8.4.12. TAXUS CARDIUM PHARMACEUTICALS GROUP, INC. (GENE BIOTHERAPEUTICS)
8.4.12.1. Overview
8.4.12.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.12.3. Product Benchmarking
8.4.12.4. Strategic Initiatives
8.4.13. UNIQURE N.V.
8.4.13.1. Overview
8.4.13.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.13.3. Product Benchmarking
8.4.13.4. Strategic Initiatives
8.4.14. TAKEDA PHARMACEUTICAL COMPANY LIMITED
8.4.14.1. Overview
8.4.14.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.14.3. Product Benchmarking
8.4.14.4. Strategic Initiatives
8.4.15. CELLECTIS S.A.
8.4.15.1. Overview
8.4.15.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.15.3. Product Benchmarking
8.4.15.4. Strategic Initiatives
8.4.16. SANGAMO THERAPEUTICS, INC.
8.4.16.1. Overview
8.4.16.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.16.3. Product Benchmarking
8.4.16.4. Strategic Initiatives
8.4.17. ORCHARD THERAPEUTICS
8.4.17.1. Overview
8.4.17.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.17.3. Product Benchmarking
8.4.17.4. Strategic Initiatives
8.4.18. GILEAD LIFESCIENCES, INC.
8.4.18.1. Overview
8.4.18.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.18.3. Product Benchmarking
8.4.18.4. Strategic Initiatives
8.4.19. BENITEC BIOPHARMA
8.4.19.1. Overview
8.4.19.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.19.3. Product Benchmarking
8.4.19.4. Strategic Initiatives
8.4.20. SIBIONO GENETECH CO., LTD
8.4.20.1. Overview
8.4.20.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.20.3. Product Benchmarking
8.4.20.4. Strategic Initiatives
8.4.21. SHANGHAI SUNWAY BIOTECH CO., LTD.
8.4.21.1. Overview
8.4.21.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.21.3. Product Benchmarking
8.4.21.4. Strategic Initiatives
8.4.22. GENSIGHT BIOLOGICS S.A.
8.4.22.1. Overview
8.4.22.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.22.3. Product Benchmarking
8.4.22.4. Strategic Initiatives
8.4.23. TRANSGENE
8.4.23.1. Overview
8.4.23.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.23.3. Product Benchmarking
8.4.23.4. Strategic Initiatives
8.4.24. CALIMMUNE, INC
8.4.24.1. Overview
8.4.24.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.24.3. Product Benchmarking
8.4.24.4. Strategic Initiatives
8.4.25. EPEIUS BIOTECHNOLOGIES CORP.
8.4.25.1. Overview
8.4.25.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.25.3. Product Benchmarking
8.4.25.4. Strategic Initiatives
8.4.26. ASTELLAS PHARMA INC
8.4.26.1. Overview
8.4.26.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.26.3. Product Benchmarking
8.4.26.4. Strategic Initiatives
8.4.27. AMERICAN GENE TECHNOLOGIES
8.4.27.1. Overview
8.4.27.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.27.3. Product Benchmarking
8.4.27.4. Strategic Initiatives
8.4.28. BIOMARIN PHARMACEUTICALS, INC
8.4.28.1. Overview
8.4.28.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
8.4.28.3. Product Benchmarking
8.4.28.4. Strategic Initiatives
List of Tables
Table 1 List of Secondary Sources
Table 2 List of Tables
Table 3 List of Secondary Sources
Table 4 List of Abbreviations
Table 5 Global Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 6 Global Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 7 Global Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 8 Global Gene Therapy Market by Region, 2018 - 2030 (USD Million)
Table 9 North America Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 10 North America Gene Therapy Market by Country, 2018 - 2030 (USD Million)
Table 11 North America Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 12 North America Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 13 U.S. Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 14 U.S. Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 15 U.S. Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 16 Canada Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 17 Canada Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 18 Canada Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 19 Europe Gene Therapy Market by Country, 2018 - 2030 (USD Million)
Table 20 Europe Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 21 Europe Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 22 Europe Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 23 Germany Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 24 Germany Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 25 Germany Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 26 UK Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 27 UK Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 28 UK Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 29 France Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 30 France Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 31 France Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 32 Italy Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 33 Italy Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 34 Italy Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 35 Spain Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 36 Spain Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 37 Spain Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 38 Asia Pacific Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 39 Asia Pacific Gene Therapy Market by Country, 2018 - 2030 (USD Million)
Table 40 Asia Pacific Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 41 Asia Pacific Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 42 China Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 43 China Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 44 China Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 45 Japan Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 46 Japan Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 47 Japan Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 48 South Korea Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 49 South Korea Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 50 South Korea Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 51 Australia Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 52 Australia Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 53 Australia Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
Table 54 RoW Gene Therapy Market by Vector Type, 2018 - 2030 (USD Million)
Table 55 RoW Gene Therapy Market by Route Of Administration, 2018 - 2030 (USD Million)
Table 56 RoW Gene Therapy Market by Indication, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Information Procurement
Fig. 2 Primary Research Pattern
Fig. 3 Market Research Approaches
Fig. 4 Value Chain-Based Sizing & Forecasting
Fig. 5 Market Formulation & Validation
Fig. 6 Gene Therapy Market Segmentation
Fig. 7 Market Driver Relevance Analysis (Current & Future Impact)
Fig. 8 Market Restraint Relevance Analysis (Current & Future Impact)
Fig. 9 Market Challenge Relevance Analysis (Current & Future Impact)
Fig. 10 Penetration & Growth Prospect Mapping
Fig. 11 SWOT Analysis, By Factor (Political & Legal, Economic and Technological)
Fig. 12 Porter’s Five Forces Analysis
Fig. 13 Regional Marketplace: Key Takeaways
Fig. 14 Global Gene Therapy Market for Lentivirus, 2018 - 2030 (USD Million)
Fig. 15 Global Gene Therapy Market for AAV, 2018 - 2030 (USD Million)
Fig. 16 Global Gene Therapy Market for RetroVirus & gamma RetroVirus, 2018 - 2030 (USD Million)
Fig. 17 Global Gene Therapy Market for Modified Herpes Simplex Virus, 2018 - 2030 (USD Million)
Fig. 18 Global Gene Therapy Market for Adenovirus, 2018 - 2030 (USD Million)
Fig. 19 Global Gene Therapy Market for Non-viral Plasmid Vector, 2018 - 2030 (USD Million)
Fig. 20 Global Gene Therapy Market for Other Vector Type, 2018 - 2030 (USD Million)
Fig. 21 Global Gene Therapy Market for Acute Lymphoblastic Leukemia (ALL), 2018 - 2030 (USD Million)
Fig. 22 Global Acute Lymphoblastic Leukemia (ALL) patients treated with gene therapy, 2018 - 2030
Fig. 23 Global Gene Therapy Market for Inherited Retinal Disease, 2018 - 2030 (USD Million)
Fig. 24 Global Inherited Retinal Disease patients treated with gene therapy, 2018 - 2030
Fig. 25 Global Gene Therapy Market for Large B-cell Lymphoma, 2018 - 2030 (USD Million)
Fig. 26 Global Large B-cell Lymphoma patients treated with gene therapy, 2018 - 2030
Fig. 27 Global Gene Therapy Market for ADA-SCID, 2018 - 2030 (USD Million)
Fig. 28 Global ADA-SCID patients treated with gene therapy, 2018 - 2030
Fig. 29 Global Gene Therapy Market for Melanoma (lesions), 2018 - 2030 (USD Million)
Fig. 30 Global Melanoma (lesions) patients treated with gene therapy, 2018 - 2030
Fig. 31 Global Gene Therapy Market for Beta-Thalassemia Major/SCD, 2018 - 2030 (USD Million)
Fig. 32 Global Beta-Thalassemia Major/SCD patients treated with gene therapy, 2018 - 2030
Fig. 33 Global Gene Therapy Market for Head & Neck Squamous Cell Carcinoma, 2018 - 2030 (USD Million)
Fig. 34 Global Head & Neck Squamous Cell Carcinoma patients treated with gene therapy, 2018 - 2030
Fig. 35 Global Gene Therapy Market for Peripheral arterial disease, 2018 - 2030 (USD Million)
Fig. 36 Global Peripheral arterial disease patients treated with gene therapy, 2018 - 2030
Fig. 37 Global Gene Therapy Market for Spinal Muscular Atrophy (SMA), 2018 - 2030 (USD Million)
Fig. 38 Global Spinal Muscular Atrophy (SMA) patients treated with gene therapy, 2018 - 2030
Fig. 39 Global Gene Therapy Market for Others, 2018 - 2030 (USD Million)
Fig. 40 Global Other diseases patient treated with gene therapy, 2018 - 2030
Fig. 41 Global Gene Therapy Market for Intravenous, 2018 - 2030 (USD Million)
Fig. 42 Global Gene Therapy Market for Others, 2018 - 2030 (USD Million)
Fig. 43 Regional Outlook, 2022 & 2030
Fig. 44 North America Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 45 U.S. Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 46 Canada Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 47 Europe Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 48 Germany Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 49 UK Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 50 France Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 51 Italy Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 52 Spain Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 53 Asia Pacific Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 54 Japan Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 55 China Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 56 Australia Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 57 South Korea Gene Therapy Market 2018 - 2030 (USD Million)
Fig. 58 Rest of the world Gene Therapy Market 2018 - 2030 (USD Million)What questions do you have? Get quick response from our industry experts. Request a Free ConsultationMarket Segmentation
- Gene Therapy Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- Gene Therapy Route of Administration Outlook (Revenue, USD Million; 2018 - 2030)
- Intravenous
- Others
- Gene Therapy Vector Type Outlook (Revenue, USD Million; 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- Gene Therapy Regional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- North America Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- North America Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- North America Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- U.S.
- U.S. Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- U.S. Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- U.S. Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- U.S. Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Canada
- Canada Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- Canada Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- Canada Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- Canada Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- North America Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Europe
- Europe Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- Europe Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- Europe Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- Germany
- Germany Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- Germany Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- Germany Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- Germany Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- UK
- UK Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- UK Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- UK Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- UK Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- France
- France Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- France Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- France Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Italy
- Italy Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- Italy Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- Italy Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- Italy Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Spain
- Spain Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- Spain Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- Spain Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- Spain Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Europe Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Asia Pacific
- Asia Pacific Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- Asia Pacific Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- Asia Pacific Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- China
- China Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- China Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- China Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- China Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Japan
- Japan Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- Japan Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- Japan Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- Japan Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Australia
- Australia Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- Australia Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- Australia Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- Australia Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- South Korea
- South Korea Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Acute Lymphoblastic Leukemia (ALL)
- Inherited Retinal Disease
- Large B-Cell Lymphoma
- ADA-SCID
- Melanoma (lesions)
- Beta-Thalassemia Major/SCD
- Head & Neck Squamous Cell Carcinoma
- Peripheral Arterial Disease
- Spinal Muscular Atrophy (SMA)
- Others
- South Korea Gene Therapy market vector type outlook (Revenue, USD Million, 2018 - 2030)
- Lentivirus
- AAV
- RetroVirus & gamma RetroVirus
- Modified Herpes Simplex Virus
- Adenovirus
- Non-viral Plasmid Vector
- Others
- South Korea Gene Therapy market route of administration outlook (Revenue, USD Million, 2018 - 2030)
- Intravenous
- Others
- South Korea Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- Asia Pacific Gene Therapy market indication outlook (Revenue, USD Million, 2018 - 2030)
- North America
Gene Therapy Market Dynamics
Driver: Robust gene therapy pipeline
Researchers are working to make gene therapy available at clinics. Although very few patients have so far received effective gene therapy treatments, gene therapy holds high potential in revolutionizing disease treatment regimens by targeting the genes responsible for disease pathogenesis. Various universities and institutes are observed to exhibit a broad portfolio of gene therapy in the pipeline, which, in turn, is expected to boost revenue generation in the near future. Clinical trials for gene therapy increased significantly from 2017 to 2018, after the FDA approved the first gene therapy. This first approval propelled the confidence of entities in the gene therapy field. Hence, robust pipeline is expected to fuel the growth of this market.
Driver: Introduction of technological advancements
Gene therapy involves the introduction of a gene of interest through recombinant DNA technology into the host’s genome to repair the mutations that underlie the disease. Few drawbacks with gene therapy pose challenges in delivering recombinant DNA into host cells. Continuous advancements in recombinant DNA technology are expected to enhance the efficiency of gene therapy in the coming years. Hence, the ongoing advancements in recombinant DNA technology are expected to expand the number of ongoing clinical trials for gene therapy. Primarily, these advancements are taking place in context of various gene-editing tools and expression systems to augment the R&D for gene therapy products.
Restraint: Absence of effective diagnosis framework
Controversies and debates over the ethics of using gene technology in human disease treatment have been increasing with the introduction of various gene-editing tools. As gene therapy involves alterations in genes, it has raised many ethical concerns. Because of these ethical issues surrounding gene therapy, the U.S. government prohibited the use of federal funds for research on germline gene therapy in people. Gene therapy allows humans to save future generations in a family from particular genetic disorders. However, it is anticipated to affect fetus development in unexpected ways. Furthermore, regulatory agencies such as the FDA have established several guidance documents demonstrating the preclinical and clinical activities as a key to regulate and review gene therapy products. The presence of stringent regulatory guidelines pertaining to the approval of gene therapy products has slowed down the growth of this market.
What Does This Report Include?
This section will provide insights into the contents included in this smart gene therapy report and help gain clarity on the structure of the report to assist readers in navigating smoothly.
Gene therapy market qualitative analysis
-
Industry overview
-
Industry trends
-
Market drivers and restraints
-
Market size
-
Growth prospects
-
Porter’s analysis
-
PESTEL analysis
-
Key market opportunities prioritized
-
Competitive landscape
-
Company overview
-
Financial performance
-
Product benchmarking
-
Latest strategic developments
-
Gene therapy market quantitative analysis
-
Market size, estimates, and forecast from 2018 to 2030
-
Market estimates and forecast for product segments up to 2030
-
Regional market size and forecast for product segments up to 2030
-
Market estimates and forecast for application segments up to 2030
-
Regional market size and forecast for application segments up to 2030
-
Company financial performance
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationResearch Methodology
A three-pronged approach was followed for deducing the gene therapy market estimates and forecasts. The process has three steps: information procurement, analysis, and validation. The whole process is cyclical, and steps repeat until the estimates are validated. The three steps are explained in detail below:
Information procurement: Information procurement is one of the most extensive and important stages in our research process, and quality data is critical for accurate analysis. We followed a multi-channel data collection process for gene therapy market to gather the most reliable and current information possible.
- We buy access to paid databases such as Hoover’s and Factiva for company financials, industry information, white papers, industry journals, SME journals, and more.
- We tap into Grand View’s proprietary database of data points and insights from active and archived monitoring and reporting.
- We conduct primary research with industry experts through questionnaires and one-on-one phone interviews.
- We pull from reliable secondary sources such as white papers and government statistics, published by organizations like WHO, NGOs, World Bank, etc., Key Opinion Leaders (KoL) publications, company filings, investor documents, and more.
- We purchase and review investor analyst reports, broker reports, academic commentary, government quotes, and wealth management publications for insightful third-party perspectives.
Analysis: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilized different methods of gene therapy market data depending on the type of information we’re trying to uncover in our research.
-
Market Research Efforts: Bottom-up Approach for estimating and forecasting demand size and opportunity, top-down Approach for new product forecasting and penetration, and combined approach of both Bottom-up and Top-down for full coverage analysis.
-
Value-Chain-Based Sizing & Forecasting: Supply-side estimates for understanding potential revenue through competitive benchmarking, forecasting, and penetration modeling.
-
Demand-side estimates for identifying parent and ancillary markets, segment modeling, and heuristic forecasting.
-
Qualitative Functional Deployment (QFD) Modelling for market share assessment.
Market formulation and validation: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilize different methods of data analysis depending on the type of information we’re trying to uncover in our research.
-
Market Formulation: This step involves the finalization of market numbers. This step on an internal level is designed to manage outputs from the Data Analysis step.
-
Data Normalization: The final market estimates and forecasts are then aligned and sent to industry experts, in-panel quality control managers for validation.
-
This step also entails the finalization of the report scope and data representation pattern.
-
Validation: The process entails multiple levels of validation. All these steps run in parallel, and the study is forwarded for publishing only if all three levels render validated results.
Gene Therapy Market Categorization:
The gene therapy market was categorized into four segments, namely indication (Acute Lymphoblastic Leukemia, Inherited Retinal Disease, Large B-cell Lymphoma, ADA-SCID, Melanoma (lesions), Beta-Thalassemia Major/SCD, Head & Neck Squamous Cell Carcinoma, Peripheral arterial disease, Spinal Muscular Atrophy), vector type (Lentivirus, AAV, RetroVirus & gamma RetroVirus, Modified Herpes Simplex Virus, Adenovirus, Non-viral Plasmid Vector), route of administration (Intravenous), and regions (North America, Europe, Asia Pacific, Rest of the world).
Segment Market Methodology:
The gene therapy market was segmented into indication, vector type, route of administration, and regions. The demand at a segment level was deduced using a funnel method. Concepts like the TAM, SAM, SOM, etc., were put into practice to understand the demand. We at GVR deploy three methods to deduce market estimates and determine forecasts. These methods are explained below:
Market research approaches: Bottom-up
-
Demand estimation of each product across countries/regions summed up to from the total market.
-
Variable analysis for demand forecast.
-
Demand estimation via analyzing paid database, and company financials either via annual reports or paid database.
-
Primary interviews for data revalidation and insight collection.
Market research approaches: Top-down
-
Used extensively for new product forecasting or analyzing penetration levels.
-
Tool used invoice product flow and penetration models Use of regression multi-variant analysis for forecasting Involves extensive use of paid and public databases.
-
Primary interviews and vendor-based primary research for variable impact analysis.
Market research approaches: Combined
- This is the most common method. We apply concepts from both the top-down and bottom-up approaches to arrive at a viable conclusion.
Regional Market Methodology:
The gene therapy market was analyzed at a regional level. The globe was divided into North America, Europe, Asia Pacific, Rest of the world, keeping in focus variables like consumption patterns, export-import regulations, consumer expectations, etc. These regions were further divided into Eleven countries, namely, the U.S., Canada, the UK, Germany, France, Italy, Spain, Japan, China, South Korea, Australia
All three above-mentioned market research methodologies were applied to arrive at regional-level conclusions. The regions were then summed up to form the global market.
Gene therapy market companies & financials:
The gene therapy market was analyzed via companies operating in the sector. Analyzing these companies and cross-referencing them to the demand equation helped us validate our assumptions and conclusions. Key market players analyzed include:
-
RegenxBio, Inc.: RegenxBio, Inc. was founded in 2008 and is headquartered in Maryland, U.S. It is a biotechnology company focused on the development of recombinant Adeno Associated Virus (AAV) gene therapy. The company has its own technology platform that holds exclusive rights to more than 100 AAV vectors including NAV Vectors. REGENXBIO is planning to build internal gene therapy departments and develop multiple product candidates for metabolic, retinal therapeutics, and neurodegenerative areas.
-
Oxford BioMedica plc: Oxford BioMedica (OXB) plc was founded in 1995 and is headquartered at Oxford, UK. The company is focused on manufacturing gene-altering molecules to develop a cure for rare diseases. OXB developed an exclusive LentiVector platform, which might help in developing single-dose genetic cure for rare diseases. Through the LentiVector technology platform, the company is developing various gene therapy molecules to treat various ailments such as various types of cancer, ophthalmic diseases, and many CNS disorders.
-
Voyager Therapeutics: Voyager Therapeutics was established in 2013 and is headquartered in Massachusetts, U.S. The company is involved in developing genetically modified treatments for diseases and disorders associated with the central nervous system. Voyager is focused on developing advanced AAV gene therapy by the process of vector engineering, optimization, and development of AAV vectors.
-
Human Stem Cells Institute: Human Stem Cells Institute was founded in 2003 and is headquartered in Moscow, Russia. The company is focuses on the development and commercialization of stem cell technologies to cure rare genetic diseases. The company received international attention following the launch of the gene therapy drug, Neovasculgen, an innovative drug used as curative for the Peripheral Arterial Disease (PAD) and Critical Limb Ischemia (CLI) caused by atherosclerosis.
-
Dimension Therapeutics. Inc.: Dimension Therapeutics. Inc. was founded in 2013 and is headquartered in Massachusetts, U.S. The company is focused on the development of novel gene therapeutics for severe and rare genetic disorders including Ornithine Transcarbamylase (OTC) deficiency, glycogen storage disease type Ia, and hemophilia. The company operates as a subsidiary of Ultragenyx Pharmaceutical after its acquisition by Ultragenyx in November 2017.
-
Bristol-Myers Squibb Company: Bristol-Myers Squibb Company was founded in 1897 and is headquartered in New York, U.S. This biopharmaceutical company has delivered innovative medicines to aid patients in prevailing over serious diseases. Many drugs developed by them helped millions of patients fight against life-threatening diseases, such as cancer, cardiovascular disease, hepatitis B, and HIV.
-
Sanofi: Sanofi was founded in 2004 and is headquartered in Paris, France. This is a life sciences company focused on improving healthcare access and providing support to people through their innovative and safe drugs. The company transformed healthcare from prevention to treatment by scientific innovation into various types of healthcare solutions. It has expertise in the fields of human vaccines, multiple sclerosis, rare diseases, immunology, oncology, infectious diseases, cardiovascular solutions, and diabetes.
-
Applied Genetic Technologies Corporation: Founded in 1999 and headquartered in Florida, U.S, AGTC is a biotechnology company that has its own gene therapy platform for the development of rare inherited diseases in the field of ophthalmology. The company focuses on rare and genetic diseases of the eye, which occur due to the mutations in genes that can affect vision. It has expertise in capsids and expression cassettes including the design, development, and expression of gene therapy products. In October 2022, AGTC was acquired by Syncona.
-
F. Hoffmann-La Roche Ltd.: F. Hoffmann-La Roche Ltd. was founded in 1896 and is headquartered in Basel, Switzerland. The company develops and manufactures diagnostic & pharmaceutical products. It has 26 manufacturing sites across the globe with two business segments: pharmaceuticals and diagnostics. The pharmaceutical segment manufactures drugs for infectious diseases, cardiovascular, respiratory, & metabolic disorders, cancer, and central nervous system & autoimmune diseases.
-
bluebird Bio, Inc.: bluebird Bio, Inc. was founded in 1992 and is headquartered in Massachusetts, U.S. It is one of the few biotechnological companies leading in the gene therapy revolution. The company’s product platforms improve cancer immunotherapy, gene therapy, and gene editing. These platforms are likely to provide potential treatment or cure for a broad range of rare genetic diseases. With its extensive developmental methods, the company aims to develop a complication-free single-dose treatment option for patients suffering from severe genetic & rare diseases as well as cancer. As of January 31, 2022, the company had 518 full-time employees.
Value chain-based sizing & forecasting
Supply Side Estimates
-
Company revenue estimation via referring to annual reports, investor presentations, and Hoover’s.
-
Segment revenue determination via variable analysis and penetration modeling.
-
Competitive benchmarking to identify market leaders and their collective revenue shares.
-
Forecasting via analyzing commercialization rates, pipelines, market initiatives, distribution networks, etc.
Demand side estimates
-
Identifying parent markets and ancillary markets
-
Segment penetration analysis to obtain pertinent
-
revenue/volume
-
Heuristic forecasting with the help of subject matter experts
-
Forecasting via variable analysis
Gene Therapy Market Report Objectives:
-
Understanding market dynamics (in terms of drivers, restraints, & opportunities) in the countries.
-
Understanding trends & variables in the individual countries & their impact on growth and using analytical tools to provide high-level insights into the market dynamics and the associated growth pattern.
-
Understanding market estimates and forecasts (with the base year as 2023, historic information from 2018 to 2023, and forecast from 2024 to 2030). Regional estimates & forecasts for each category are available and are summed up to form the global market estimates.
Gene Therapy Market Report Assumptions:
-
The report provides market value for the base year 2023 and a yearly forecast till 2030 in terms of revenue/volume or both. The market for each of the segment outlooks has been provided on region & country basis for the above-mentioned forecast period.
-
The key industry dynamics, major technological trends, and application markets are evaluated to understand their impact on the demand for the forecast period. The growth rates were estimated using correlation, regression, and time-series analysis.
-
We have used the bottom-up approach for market sizing, analyzing key regional markets, dynamics, & trends for various products and end-users. The total market has been estimated by integrating the country markets.
-
All market estimates and forecasts have been validated through primary interviews with the key industry participants.
-
Inflation has not been accounted for to estimate and forecast the market.
-
Numbers may not add up due to rounding off.
-
Europe consists of EU-8, Central & Eastern Europe, along with the Commonwealth of Independent States (CIS).
-
Asia Pacific includes South Asia, East Asia, Southeast Asia, and Oceania (Australia & New Zealand).
-
Latin America includes Central American countries and the South American continent
-
Middle East includes Western Asia (as assigned by the UN Statistics Division) and the African continent.
Primary Research
GVR strives to procure the latest and unique information for reports directly from industry experts, which gives it a competitive edge. Quality is of utmost importance to us, therefore every year we focus on increasing our experts’ panel. Primary interviews are one of the critical steps in identifying recent market trends and scenarios. This process enables us to justify and validate our market estimates and forecasts to our clients. With more than 8,000 reports in our database, we have connected with some key opinion leaders across various domains, including healthcare, technology, consumer goods, and the chemical sector. Our process starts with identifying the right platform for a particular type of report, i.e., emails, LinkedIn, seminars, or telephonic conversation, as every report is unique and requires a differentiated approach.
We send out questionnaires to different experts from various regions/ countries, which is dependent on the following factors:
-
Report/Market scope: If the market study is global, we send questionnaires to industry experts across various regions, including North America, Europe, Asia Pacific, Latin America, and MEA.
-
Market Penetration: If the market is driven by technological advancements, population density, disease prevalence, or other factors, we identify experts and send out questionnaires based on region or country dominance.
The time to start receiving responses from industry experts varies based on how niche or well-penetrated the market is. Our reports include a detailed chapter on the KoL opinion section, which helps our clients understand the perspective of experts already in the market space.
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationShare this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





