- Home
- »
- Clinical Diagnostics
- »
-
Hemato Oncology Testing Market Size & Share Report, 2030GVR Report cover
![Hemato Oncology Testing Market Size, Share & Trends Report]()
Hemato Oncology Testing Market Size, Share & Trends Analysis Report By Product (Assay Kits & Reagents, Services), By Cancer Type (Leukemia, Lymphoma), By Technology, By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68039-174-2
- Number of Pages: 150
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
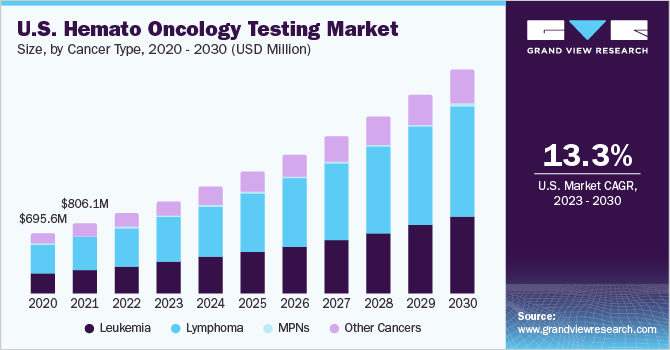
The global hemato oncology testing market size was estimated at USD 3.07 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 12.5% from 2023 to 2030. Hemato oncology is associated with the treatment, diagnosis, and prevention of blood-related cancers and diseases. The growing prevalence of myeloma and lymphoma along with increased funding in research and development (R&D) and accessibility of advanced technology products are anticipated to boost the growth of the market during the forecast period.
The market for hemato oncology testing has been significantly impacted by the COVID-19 pandemic. Due to the overwhelming focus on managing and treating COVID-19 patients, non-essential medical procedures, including cancer screening and testing, were delayed, or deferred. This has resulted in a decline in the demand for hemato oncology testing during the pandemic. In addition, disruptions in the supply chain and healthcare infrastructure have further affected the market. However, with the gradual recovery and resumption of routine healthcare services, the market is expected to regain momentum in the post-pandemic period.
According to the American Cancer Society, Non-Hodgkin Lymphoma (NHL) is one of the most common forms of cancer in the U.S., accounting for approximately 4% of all cancers in the country. It is estimated that approximately 80,550 individuals, 44,880 men, and 35,670 women, were estimated to be diagnosed with NHL in 2023. Furthermore, approximately 20,180 (11,780 men and 8,400 women) individuals were expected to die due to NHL in 2023. Approximately 1 out of 43 males and 1 out of 53 females are estimated to develop NHL during their lifetime.
Similarly, as per the U.S. National Institutes of Health (NIH), NHL is the seventh most common cancer in the U.S., representing 4.3% of all cancers in the nation. In addition, the median age of people most diagnosed with NHL is 67 years. With the use of statistical models, NIH estimated that in 2023 the new cases of NHL in the U.S. would be approximately 80,550, and the number of people dying from the disease would be approximately 20,180. Furthermore, the mortality rate has also decreased by an average of 2.1% each year from 2017 to 2019. Timely diagnosis and effective treatment plans are among the major factors contributing to the decline in incidence and mortality rates.
Personalized medicine promises a paradigm shift in diagnosis and care delivery as treatment planning is based on data obtained through a holistic approach. The proliferation of sequencing methodologies, especially next-generation sequencing (NGS), due to the rising cost of sequencing and the development of the Human Genome Project in the field of genomics is expected to drive the hemato oncology testing market’s growth.
The introduction of innovative molecular techniques has been important in addressing matters relating to blood cancer diagnosis and treatment. The use of immunophenotyping in the detection of acute myeloid leukemia (AML) is one such example. This technique relies on the evaluation of leukocyte cancer cells and aids in the characterization of cancer types. Similarly, chronic myelogenous leukemia (CML) can be diagnosed with cytogenetic techniques such as PCR targeted at ABL/BCR genes or FISH. International standardization of BCR/ABL measures has facilitated laboratories to easily diagnose blood cancers.
The increasing availability of RT-PCR analysis for detecting cancers such as CML, AML, and acute lymphocytic leukemia (ALL) has been vital in increasing the adoption of molecular diagnostic testing services, facilitating the market growth. An increase in the co-development of the drug diagnostics model is also expected to fuel the growth of the market during the forecast period. For instance, in November 2023, FDA approved the IDH1 CDx Test for the detection and treatment of AML patients. Similarly, in the year 2018, Invivoscribe announced the U.S. FDA approval for LeukoStrat CDx FLT3 Mutation Assay and XOSPATA, an AML drug manufactured by Astellas Pharma.
Cancer Type Insights
In terms of cancer type, the lymphoma segment dominated the market in 2022 with the largest revenue share of 46.66% and is projected to witness the fastest CAGR of 13.0% over the forecast period owing to the growing prevalence of Hodgkin and non-Hodgkin lymphoma globally. It is estimated that approximately 80,550 individuals, 44,880 men, and 35,670 women, were estimated to be diagnosed with NHL in 2023. Furthermore, approximately 20,180 (11,780 men and 8,400 women) individuals were expected to die due to NHL in 2023. Similarly, the data from Cancer Research UK indicates that the incidence of NHL is rising, with 13,900 new cases of NHL being diagnosed each year in the UK.
Thus, various private and public organizations are undertaking initiatives to enhance awareness among individuals globally. For instance, in March 2023, Royalty Pharma plc announced a contribution of USD 7.5 million to The Leukemia & Lymphoma Society. This aims to support initiatives aimed at reducing healthcare discrepancies in blood cancer testing and treatment.
A rise in the availability of technologically advanced products for the diagnosis of AML is also anticipated to fuel the growth of the market during the forecast period. As the diagnosis of leukemia takes a prolonged time, the growing integration of machine learning is expected to boost market growth over the forecast period. For instance, in September 2020, The University of Bonn has shown that artificial intelligence could be used for the diagnosis of leukemia. When compared to current methods, this technology maximizes the use of measurement values, improving both the speed and objectivity of studies. Smaller laboratories can now take advantage of this machine learning approach that is publicly available thanks to recent developments that have made it more affordable. In terms of its use in clinical practice, this is a considerable advancement.
Product Insights
In terms of product, the services segment dominated the hemato oncology testing market in 2022 and held the largest revenue share of 58.23% owing to the increasing prevalence of myeloma, NHL, and leukemia. Growing awareness about several treatment therapies including personalized medicines is also expected to drive segment growth during the forecast period. Companies are collaborating to expand their genomic testing portfolio and provide standardized tests globally. For instance, in April 2023, Illumina collaborated with Kartos Therapeutics to increase the usage of its genomic profiling test for blood cancers. The company aims to deliver a standardized, globally distributable test for precision oncology providers.
The services segment is estimated to grow at the fastest CAGR of 13.0% over the forecast period. The advancements in the development of new tests for myeloma diagnosis are also key factor contributing to the growth of the market in the coming years. For instance, in October 2023, Roche launched AVENIO Tumor Tissue CGP Kit a comprehensive genomic profiling kit to increase access to personalized cancer research. These latest launches and developments in the field of genomic science will further increase the usage of assay kits and reagents.
Technology Insights
Based on technology, the PCR segment dominated the market in 2022 with the largest revenue share of 31.08%. This is owing to its ease of use, efficiency, accuracy affordability, and easy availability.The use of PCR has been key in the diagnosis of blood cancers, as these tests are highly sensitive and detect the presence of various biomarkers in bone marrow and blood cells. PCR is highly efficient in the detection of blood cancer cells that go undetectable by cytogenic techniques, such as FISH.
In 2023, quantitative RT-PCR was used to monitor disease-associated clonal markers post-therapy, which has revolutionized how we evaluate disease response and predict outcomes. The proper identification of measurable residual disease (MRD) monitoring in ALL using T-cell receptor and immunoglobin genes is an important prognostic indicator that is used for treatment stratification.
The NGS segment is expected to witness the fastest CAGR of 13.8% during the forecast period due to various advantages offered by this technology such as more sensitivity and specificity.Several cancer researchers have deemed the substantial importance of this technology in hemato-oncology and have put efforts to develop bioinformatics algorithms that could be utilized for cancer screening and test development. For instance, in December 2023, Huntsman Cancer Institute at the University of Utah stated that NGS-based detection has shown a significant potential to predict the risk of relapse of MRD in young adults and children.
End-use Insights
In terms of end-use, hospitals captured the highest market share of 75.07% in 2022 owing to the rise in the incidence rate of blood cancers, various diagnostic tests provided, and the presence of healthcare professionals to conduct diagnostic tests and monitor the disease availability of specialized laboratory settings. According to the data published by Statista, approximately 900.6 million outpatient department visits were recorded in the U.S.
In addition, there is a significant demand for diagnosis of blood cancers such as leukemia, lymphoma, and other blood-related disorders, such as thalassemia & hemophilia, facilitating increasing demand for the same. Hospitals are major care centers for diagnosis and treatment, with the majority of diagnostic tests being conducted from independent clinics or in-house departments. Hence, the hospital segment is expected to grow at the fastest CAGR of 12.8% during the forecast period.
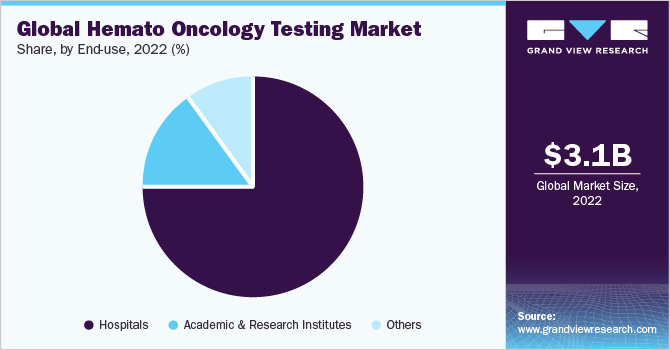
The academic and research institutes segment accounted for a significant revenue share in 2022 due to the growing number of research institutes and laboratories globally while conducting various research studies to enhance the hemato-oncology field. The rising demand for standardization in techniques, such as dPCR, is likely to boost the adoption over the coming years. Moreover, increased funding to the public, as well as private research organizations, is expected to further augment the demand for hemato-oncology products.
Regional Insights
North America dominated the hemato oncology testing market in 2022 with the largest revenue share of 37.15%. This growth is attributed to the rising incidence of leukemia, lymphoma, and multiple myeloma. Moreover, the availability of advanced healthcare and medical infrastructure and the introduction of advanced technologies have supported the region’s market growth.
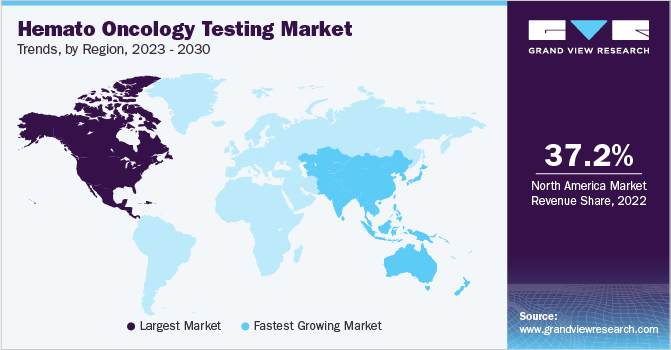
On the other hand, the Asia Pacific region is anticipated to register the fastest CAGR over the forecast period. The growing awareness among the population regarding the early diagnosis of cancer and other diseases is anticipated to fuel the regional market growth. Recent approval and commercialization of novel tests in the countries, growing awareness & adoption of testing, supportive government regulations, and high prevalence of blood cancers are likely to drive the market over the coming years. Furthermore, the entry of market players in the Asia Pacific region is expected to boost market growth during the forecast period.
Key Companies & Market Share Insights
A rise in collaborations among various market players is anticipated to fuel the growth of the market during the forecast period. For instance, in November 2020, Adaptive Biotechnologies collaborated with GSK to use its clonoSEQ assay to assess MRD in GlaxoSmithKline plc’s range of hematology products. This collaboration enhanced the company’s market position. Some of the prominent players in the global hemato oncology testing market include:
-
F. Hoffmann-La Roche Ltd
-
Abbott
-
EntroGen, Inc.
-
Qiagen N.V.
-
Cepheid
-
Thermo Fisher Scientific, Inc.
-
Bio-Rad Laboratories, Inc.
-
Illumina, Inc.
-
Amoy Diagnostics Co. Ltd.;
-
ASURAGEN, INC
-
ArcherDX, Inc.
Hemato Oncology Testing Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 3.50 billion
Revenue forecast in 2030
USD 8.00 billion
Growth rate
CAGR of 12.54% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
August 2023
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Cancer type, product, technology, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; Russia; China; Japan; India; Australia; South Korea; Singapore; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
F. Hoffmann-La Roche Ltd; Abbott; EntroGen, Inc.; Qiagen N.V.; Cepheid; Thermo Fisher Scientific, Inc.; Bio-Rad Laboratories, Inc.; Illumina, Inc; Amoy Diagnostics Co. Ltd.; ASURAGEN, INC; ArcherDX, Inc.
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Global Hemato Oncology Testing Market Report Segmentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the global hemato oncology testing market report based on cancer type, product, technology, end-use, and region:
-
Cancer Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Leukemia
-
Acute Myeloid Leukemia (AML)
-
Acute Lymphocytic Leukemia (ALL)
-
Chronic Lymphocytic Leukemia
-
Chronic Myeloid Leukemia
-
-
Lymphoma
-
Non-Hodgkin Lymphoma
-
Hodgkin Lymphoma
-
-
Myeloproliferative Neoplasms
-
Polycythemia vera (PV)
-
Essential thrombocythemia (ET)
-
Myelofibrosis (MF)
-
-
Other Cancers
-
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Assay Kits and Reagents
-
Services
-
-
Technology Outlook (Revenue, USD Million, 2018 - 2030)
-
PCR
-
Real-time qPCR
-
Digital PCR
-
-
IHC
-
NGS
-
Cytogenetics
-
Other Technologies
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Academic & Research Institutes
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Denmark
-
Norway
-
Russia
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Singapore
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. Some key players operating in the hemato oncology testing market include Cepheid, Amoy Diagnostics Co., Ltd., EntroGen, Inc., Asuragen, Inc., and ArcherDX, Inc.
b. Key factors that are driving the hemato oncology testing market growth include the rising prevalence of blood cancer, increasing drug diagnostics co-development, and the availability of advanced molecular diagnostics techniques for hemato oncology testing.
b. The global hemato oncology testing market size was estimated at USD 3.07 billion in 2022 and is expected to reach USD 3.50 billion in 2023.
b. The global hemato oncology testing market is expected to grow at a compound annual growth rate of 12.54% from 2023 to 2030 to reach USD 8.00 billion by 2030.
b. North America dominated the hemato oncology testing market with a share of 37.15% in 2022. This is attributable to the rising prevalence of lymphoma and myeloma and the growing demand for personalized therapy.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





