- Home
- »
- Pharmaceuticals
- »
-
Hemophilia Market Size, Share And Growth Report, 2030GVR Report cover
![Hemophilia Market Size, Share & Trends Report]()
Hemophilia Market Size, Share & Trends Analysis Report By Type (Hemophilia A), By Treatment Type (On-demand), By Therapy (Gene Therapy & Monoclonal Antibodies), By Distribution Channel, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: 978-1-68038-989-0
- Number of Pages: 105
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
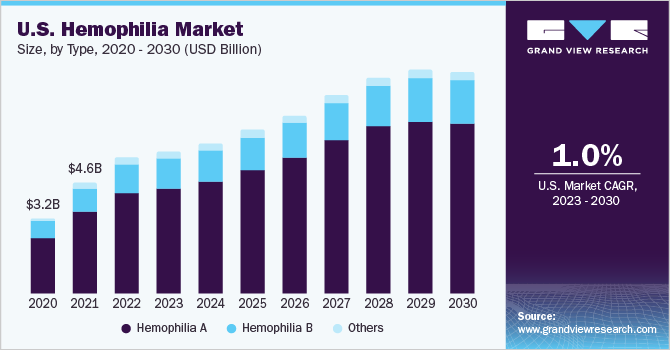
The global hemophilia market size was estimated at USD 12.6 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 6.6% from 2023 to 2030. The major factors driving the market are gene therapy approval for hemophilia treatment and the presence of multiple other candidates in various pipeline phases. Moreover, growing awareness of hemophilia disorders and increasing government support initiatives for early detection in neonates are anticipated to fuel market growth. COVID-19 pandemic-imposed restrictions had far-reaching consequences on patients as they faced difficulty accessing healthcare, hindered supply chain for treatments, and declined market demand. However, by January 2021, the market resumed normalcy of demand and supply. Based on the data published in October 2021 by the World Federation of Hemophilia (WFH), disorder occurrence is more in males than females due to sex linkage. In 2020, the WFH survey reported 209,614 people suffering from hemophilia globally, including 165,379 patients with type A, 33,076 patients with type B, and 11,159 patients with the unknown type. Thus, the disorder's high prevalence has catered to the rising adoption of therapies and driving the market growth.
Furthermore, increasing financial support for R&D through investments and the growing grants for research are boosting market growth. For instance, in March 2022, the Indiana University School of Medicine successfully raised USD 12 million of funding from the National Heart Blood and Lung Institute to develop improved therapies for the condition. R&D is stepping towards a better treatment approach using gene therapy and monoclonal antibodies. For instance, in April 2020, the FDA approved the genetically engineered product Sevenfact [coagulation factor VIIa (recombinant)-jncw] as a treatment option to control bleeding episodes in adolescents 12 years and above and adults. Such product approvals are anticipated to propel the market growth.
Type Insights
Hemophilia A held the highest share of 74.16% in 2022 in the hemophilia market. It is a genetic disorder that causes a lack of blood clotting factor VIII. The factors contributing to the dominance are the high prevalence of hemophilia A in developed regions and supportive government initiatives to launch products in major markets such as the U.S., Europe, and Japan. According to WFH’s survey in 2020, countries like the U.S., India, and Brazil are leading with 10,000 type A cases.
Hemophilia B is expected to expand at a CAGR of 5.5% during the forecast period. A strong pipeline of products and the launching of gene therapy have catered to market growth. For instance, in February 2023, the European Commission granted conditional approval to CSL Behring for its gene therapy Hemgenix for the treatment of hemophilia B. The product has been approved in the U.S. and is available at a price point of USD 3.5 million for a one-time treatment.
Treatment Insights
The prophylaxis segment dominated the hemophilia market with a share of 48.8% in 2022. Prophylaxis treatments are generally prescribed for severe patients. A novel approach to treatment for regular infusion of clotting factor concentrates is leading to segment growth. For instance, increasing the utilization of the bi-specific antibody product Helimbra (emicizumab) to treat the deficiency of clotting factor FVIII is driving the market growth. Moreover, such type of treatments is expected to result in a better quality of life due to reduced productivity loss.
The cure segment is positioned to expand at the fastest CAGR of 205.8% during the forecast period owing to the approval of multiple gene therapy products. As of June 2023, only one gene therapy product Hemgenix, has been approved both in Europe and U.S. Pfizer’s PF-07055480 (giroctocogene fitelparovec) and PF-06838435/ fidanacogene elaparvovec are in phase 3 trials for type A & B respectively. BioMarin’s ROCTAVIAN a gene therapy for type A has been approved in Europe in August 2022.
Therapy Insights
The factor replacement therapy segment held the highest share of 61.9% in 2022 in the hemophilia market. Factor replacement therapy is regarded as a standard treatment option that enables the replacement of missing clotting factors in patients with both type A & B. Factor VIII replacement products are used for type A patients whereas Factor IX replacement products are used for type B patients. Factor replacement products can either be plasma-derived or produced using recombinant DNA technology.
The emerging gene therapy and monoclonal antibodies segment is expected to expand at a CAGR of 10.1% during the forecast period. The rich gene therapy clinical pipeline coupled with the success of Helimbra (emicizumab), a bispecific antibody that works by replacing the function of VIII in the blood clotting process. The monoclonal antibodies currently under late-stage clinical trials are Pfizer’s marstacimab (phase 3) and Novo Nordisk’s NNC0365-3769 (phase3).
Distribution Channel Insights
The specialty pharmacies segment dominated the hemophilia market with a share of 61.4% in 2022. Hemophilia is a rare and complex disorder, there are very few trained professionals that can offer hemophilia treatment. Most of the hemophilia patients in the U.S. receive treatment at the “hemophilia treatment centers” or HTCs as they are popularly known. Most of the HTCs have multidisciplinary teams that are experts in managing hemophilia and receive most of their drug requirements from specialty pharmacies.
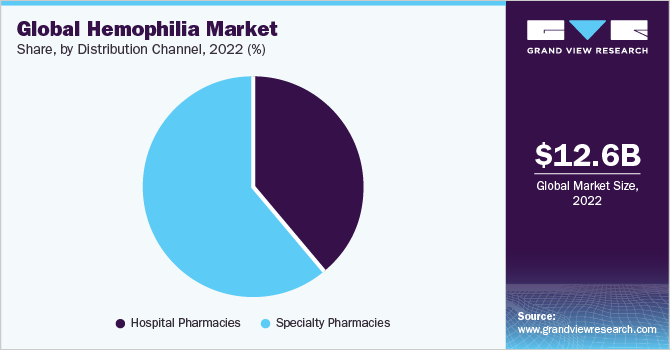
The hospital pharmacies segment is expected to expand at the fastest CAGR of 7.1% during the forecast period. The approval of gene therapy which would mostly be administered in hospitals as it requires continuous monitoring for side effects is expected to drive segment growth. Additionally, some of the HTCs are also associated with hospitals.
Regional Insights
North America was the largest revenue-generating region in the hemophilia market, with a share of 49.81% in 2022, which can be attributed to the presence of key players, growing patient treatment adoption, favorable funding assistance, presence of dedicated hemophilia treatment centers, and the rising prevalence of the disorder. According to an article published by the National Hemophilia Foundation in 2021, the U.S. has around 25,000 people with hemophilia but it costs as high as USD 270,000 per claimant annually.
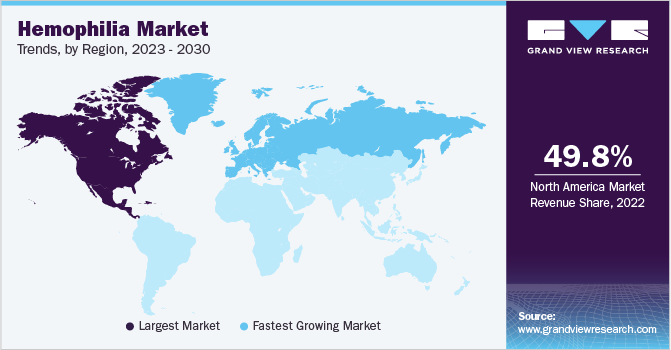
Europe is expected to grow at a CAGR of 7.4% during the forecast period due to the rising prevalence of hemophilia and regulatory support for novel product approval in the region. For instance, in February 2023, Hemgenix, a novel gene therapy, received approval from the European Commission for the treatment of hemophilia B. The product is developed and distributed by CSL Behring and manufactured by UniQure Inc. This approval is projected to initiate a key shift in the paradigm of hemophilia B patient treatment, as it relieves the burden of undergoing life-long infusions.
Key Companies & Market Share Insights
The key players in the hemophilia market are engaged in strategic collaborations, new product launches, geographical expansion, and partnerships through mergers and acquisitions in emerging and economically favorable regions. For instance, in February 2023, the U.S. FDA approved Sanofi’s once-weekly Factor VIII replacement therapy ALTUVIIIO for on-demand and prophylactic treatment of hemophilia A. Some prominent players in the global hemophiliamarket include:
-
Takeda Pharmaceutical Company Limited
-
CSL Behring
-
Pfizer, Inc.
-
Bayer AG
-
BioMarin
-
Spark Therapeutics, Inc.
-
Sanofi
-
F. Hoffmann La-Roche Ltd.
-
Novo Nordisk A/S.
-
Octapharma AG.
Hemophilia Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 13.53 billion
Revenue forecast in 2030
USD 21.07 billion
Growth rate
CAGR of 6.6% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report Updated
July 2023
Quantitative units
Revenue in USD Billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type; treatment type; therapy; distribution channel; region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Takeda Pharmaceutical Company Limited; CSL Behring; Pfizer, Inc.; Bayer AG; BioMarin, Spark Therapeutics, Inc.; Sanofi; F. Hoffmann La-Roche Ltd..; Novo Nordisk A/S.; and Octapharma AG.
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Global Hemophilia Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented the global hemophilia market report on the basis of type, treatment type, therapy, distribution channel, and region:
-
Type Outlook (Revenue, USD Billion, 2018 - 2030)
-
Hemophilia A
-
Hemophilia B
-
Others
-
-
Treatment Type Outlook (Revenue, USD Billion, 2018 - 2030)
-
On-demand
-
Cure
-
Prophylaxis
-
-
Therapy Outlook (Revenue, USD Billion, 2018 - 2030)
-
Factor Replacement Therapy
-
Plasma-derived Factor Concentrates
-
Factor VIII
- Factor IX
-
-
Recombinant Factor Concentrates
-
Factor VIII
-
Factor VII
-
Factor IX
-
-
-
Desmopressin & Fibrin Sealants
-
Gene Therapy & Monoclonal Antibodies
-
-
Distribution Channel Outlook (Revenue, USD Billion, 2018 - 2030)
-
Hospital Pharmacies
-
Specialty Pharmacies
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
- Canada
-
-
Europe
-
Germany
-
France
-
UK
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
- Kuwait
-
-
Frequently Asked Questions About This Report
b. The global hemophilia market size was estimated at USD 12.60 billion in 2022 and is expected to reach USD 13.53 billion in 2023.
b. The global hemophilia market is expected to grow at a compound annual growth rate of 6.6% from 2023 to 2030 to reach USD 21.07 billion by 2030.
b. Based on type, hemophilia A dominated the hemophilia market with a share of 74.16% in 2022. This is attributable to the target disease prevalence and the higher number of approved products.
b. Some key players operating in the hemophilia market include Takeda Pharmaceutical Company Limited; CSL Behring; Pfizer, Inc.; Bayer AG; BioMarin; Spark Therapeutics, Inc.; Sanofi; F. Hoffmann La-Roche Ltd.; Novo Nordisk A/S; and Octapharma AG.
b. Key factors that are driving the market growth include gene therapy approval for hemophilia treatment, presence of strong pipeline and the rising life expectancy due to the availability of treatments.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





