- Home
- »
- Biotechnology
- »
-
Induced Pluripotent Stem Cells Production Market Report 2030GVR Report cover
![Induced Pluripotent Stem Cells Production Market Size, Share & Trends Report]()
Induced Pluripotent Stem Cells Production Market Size, Share & Trends Analysis Report By Process, By Workflow (Reprogramming, Cell Culture), By Product, By Application (Regenerative Medicine), By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68039-549-7
- Number of Pages: 125
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Market Size & Trends
The global induced pluripotent stem cells production market size was valued at USD 1.37 billion in 2022 and is anticipated to grow at a compound annual growth rate (CAGR) of 9.3% from 2023 to 2030. The potential of stem cell-based therapies, the growing incidence of cancer, increasing capital investments for stem cell-based research, and multiple advantages of induced pluripotent stem cells (iPSCs) over embryonic stem cells (ESCs) are driving the market growth significantly. According to the report published by the American Cancer Society, in 2023, it is anticipated that there will be approximately 1.9 million fresh cases diagnosed and around 609,820 deaths due to cancer in the U.S.
The increase in research activities during the COVID-19 pandemic also boosted iPSCs-based research activities. The continuous strive by scientists to develop novel treatment and therapies to manage the SARS-CoV-2 infection have driven the demand for iPSCs as research tools. In addition, as induced pluripotent stem cells can generate physiologically similar organ models or organoids, these cells can be used to understand the pathophysiology of the virus infection in humans.
For example, in October 2022, according to an article published by the Journal of Nature Cell Biology, researchers from Japan tried to understand the role of salivary glands during COVID-19 infection. The researchers used human iPSCs to generate Human Induced Salivary Glands (hiSGs) to assess the role of salivary glands as virus reservoirs. As per the authors of this research article, hiSGs can prove to be a promising in-vitro model for future investigation. This further aids the understanding of SARS CoV-2 infection spread, thereby driving the demand for induced pluripotent stem cells as a powerful research tool in the future.
The increased number of induced pluripotent stem cells based research studies is expanding the market growth drastically. Currently, more than 120 clinical trials are underway which utilize iPSCs for disease intervention or the generation of iPSCs products. In addition, the advantages offered by induced pluripotent stem cells, such as the elimination of animal models and flexibility of ethical implications associated with embryonic stem cells further propel the market growth. This has created a favorable market for iPSCs-based therapeutics from many business entities in a wide range of applications, including searching for new drugs, modelling diseases, toxicological tests, and several others. For instance, in August 2021, Fate Therapeutics declared that FT819 had successfully treated its first patient in a clinical trial. The CAR-T cell treatment known as FT819 was created using iPSCs. FT819 is an iPSC-derived engineered CAR-T cell treatment.
Many companies and research organizations are discovering the therapeutic potency of stem cell products and targeting diseases with cell-based therapeutics. For example, in June 2021, Novo Nordisk A/S announced a global partnership with Heartseed Inc., an iPSC-producing startup in Japan. Through this collaboration, the company develops, manufactures, and commercializes HS-001, which are purified cardiomyocytes derived from iPSCs. HS-001 is an investigated cell therapy for heart failure. Additionally, in July 2022, Curi Bio announced the launch of the Mantarray platform for 3D cardiac and skeletal engineered muscle tissue (EMT) contractile activity analysis relevant to humans.
Despite the potential applications of clinical-grade autologous iPSC therapies, certain challenges and limitations still persist. The primary challenge includes the reprogramming method that is completed using retroviruses which elevates the safety issues along with permanent integration of the transgene and change of copy number into the genome of iPSC. This affects the therapeutic applications of iPSCs and hinders market growth. However, the technological advancements and increased capital investments for incorporating automation in iPSCs production, understanding the functionality using omics-based data, and robust therapy product pipeline will boost the growth and present new opportunities in the induced pluripotent stem cells production market.
The manufacturing process for the production of induced pluripotent stem cells is relatively long. The induced pluripotent stem cells may exhibit loss of heterozygosity, chromosomal instability, and genetic instability over a cultured in-vitro. Furthermore, the high costs associated with iPSC production as well as immunogenicity and heterogeneity issues also make a negative impact on market demand.
Process Insights
On the basis of process, the market is segmented into manual iPSC production process and automated iPSC production process. The manual iPSC production process segment dominated the market and accounted for the largest revenue share of 77.2% in 2022. The manual iPSC production process begins with the introduction of genes/transcription factors into rising somatic cells, a reprogramming step & selection of iPSC colonies, expansion, and passaging of iPSCs. Companies with expertise in tissue procurement, gene introduction, RNA reprogramming, and cell culture and expansion drive revenue generation in this segment. For example, REPROCELL, Inc. a Japan-based company, provides GMP iPSCs manufacturing services. The company offers to generate the GMP master cell bank of iPSCs via manufacturing processes which is consistent with global regulatory guidelines.
The automated iPSC production process segment is estimated to exhibit the fastest CAGR of 11.5 % during the forecast period. The increasing demand for stem cell-based therapies requires scaling up. This demand for generating large-scale induced pluripotent stem cells (iPSCs) in a reproducible manner with minimum alterations is propelling the adoption of automated production platforms. Most companies working on iPSCs-based therapies rely on lines of allogenic cells that are taken from multiple donors. These cells are then created into off-the-shelf products on a large scale which can be used for the treatment of patients affected with the same diseases.
Workflow Insights
Based on workflow, the market is segmented into reprogramming, cell culture, cell characterization / analysis, engineering, and others. The cell culture segment dominated the market with the largest revenue share of 37.3% in 2022. Cell culture workflow is inclusive of revenues generated from iPSC harvest products, expansion products, and differentiation products; thus contributing to the market relevance of this segment. Cell culture plays a pivotal role in various stages of induced pluripotent stem cells (iPSCs) generation and continuous investments by key players operating in this market globally have made an important contribution to the segment’s revenue generation. For example, in August 2021, HebeCell Corp. announced that it has raised USD 53 million in financing to advance its R&D program, clinical operations, and commercialization for off-the-shelf pluripotent stem cell-CAR-NK products. Additionally, costs associated with cell culture matrices, media, and regular maintenance of these cells add up to the revenue generated by this segment.
The cell characterization/analysis segment is expected to grow at the fastest CAGR of 15.6% over the forecast period, owing to factors such as the growing use of assays based on cells in drug discovery due to their consistency in producing tissue-specific results. Additionally, the segment is anticipated to grow due to the rising incidence of chronic diseases like cancer, which has seen an increase in the use of cell-based assays for detection and treatment. In 2020, Thermo Fisher acquired phitonex, inc. Through this acquisition, thermo fisher was able to enhance its image multiplexing and flow cytometry capabilities for study on protein and cell analysis.
Product Insights
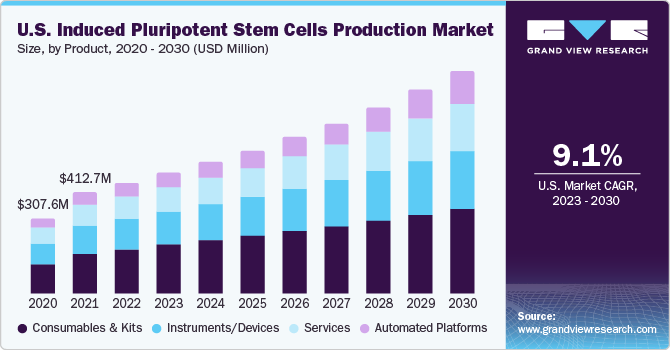
Based on product, the iPSC market is segmented into instruments/ devices, automated platforms consumables & kits, services. The consumables and kits segment dominated the market and accounted for the largest revenue share of 40.3% in 2022. The never-ceasing research and development activities in the iPSCs space are driving the demand for various consumables & kits. Multiple induced pluripotent stem cells (iPSCs) kits such as reprogramming kits, differentiation kits, and generation kits along with a selection of media & other consumables by key players are allowing end-users to utilize these products as per intended application. In addition, the utility of kits and media during toxicology testing is further propelling the growth of this product segment.
The automated platforms segment is expected to grow at the fastest CAGR of 13.5% during the forecast period, due to the demand for stem cell products, the scaling up of clinical phase iPSC therapies, and regulatory level quality control. The automated platforms provide reproducible & reliable results, reduce labor dependence, and provide well-maintained conditions of manufacturing, and standardization of protocols while eliminating human biases. For instance, Hitachi Ltd. offers an automated equipment for iPSCs culture with an aim to spread regenerative medicines. Many other companies are also offering such automated platforms to increase throughput, decrease operational costs and increase adaptability for iPSCs production.
Application Insights
On the basis of application, the market is segmented into drug development & discovery, regenerative medicine, toxicology studies, and others. The drug development & discovery segment dominated the market with the largest revenue share of 43.3% in 2022. As the world is witnessing a rise in chronic diseases, The Partnership to Fight Chronic Disease (PFCD) estimated that more than 130 million Americans are affected with chronic diseases like diabetes, asthma, heart problems, and cancer. PFCD also stated that more than 1 in 2 American adults lives with one chronic disease and about 1 in 3 suffers from two or more chronic diseases. Thus, the prevalence of diseased conditions is driving the demand for disease understanding, and induced pluripotent stem cells are widely used in disease modeling leading to the development & discovery of innovative treatment plans. For example, Edigene, Inc. and Neukio Biotherapeutics collaborated in February 2022, for development and research to create next-generation immune cell therapies using iPSC and natural killer cells (NK Cell).
The regenerative medicine segment is expected to grow at the fastest CAGR of 10.3% over the forecast period of 2023 to 2030, owing to the vast applications and robust product pipelines by companies. In addition, iPSCs can be mediated for disease-specific therapies and provide greater and safer drug development platforms. Also, iPSC-derived organoids and organs are recently being analyzed for both disease modeling and regenerative therapy. In disease modeling, induced pluripotent stem cells enable drug discovery for innovative therapeutics, and in regenerative therapy, iPSCs are proceeding with clinical trials. For example, in April 2022, Editas Medicine, Inc. announced its preclinical data (both in-vitro & in-vivo) for tumor killing capability of its NK cell therapy derived from induced pluripotent stem cells (iPSCs).
End-use Insights
Based on end use, induced pluripotent stem cells production market is segmented into research and academic institutes, biotechnology & pharmaceutical companies and hospitals & clinics. The biotechnology and pharmaceutical companies segment dominated the market with the largest revenue share of 59.3% in 2022. The availability and adoption of various kits, consumables, and instruments along with iPSCs products are driving the revenue generation in this segment. To utilize the market opportunity of the stem cell-based therapy market, biotechnology and pharmaceutical companies are boosting new product developments via intensive R&D efforts. For example, in October 2022, Fate Therapeutics announced that it will be presenting its preclinical and clinical data of multiple iPSC product platforms at Society for Immunotherapy of Cancer (SITC) meeting to be held in November 2022. This demonstrates the robust induced pluripotent stem cells (iPSCs) product pipeline in the market, further fueling the growth.
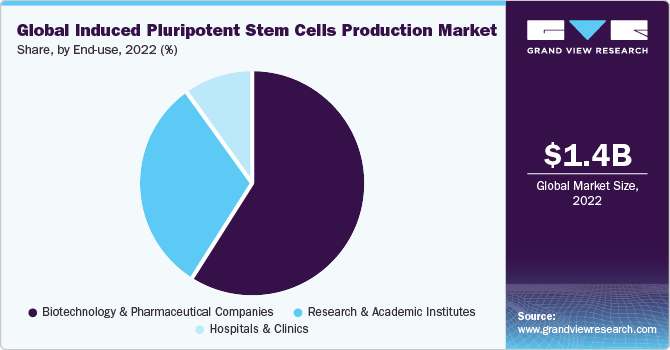
The research and academic institutes segment is expected to grow at the fastest CAGR of 11.0% over the forecast period. This is due to iPSCs having wide applications and thus being utilized in many research as well as clinical studies. The increasing utilization of iPSCs in regenerative studies provides favorable prospects for translating this technology into clinical use. For instance, in March 2022, researchers from Indiana University in collaboration with other institutions investigated the use of human induced pluripotent stem cells for the regeneration of visual acuity among diabetes patients. Furthermore, growing research setting to evaluate the safety and efficacy of iPSC-based treatments will drive market growth.
Regional Insights
North America dominated the market and accounted for the largest revenue share of 40.8% in 2022. The growing incidence of chronic diseases in this region, developed healthcare infrastructure, funds from private & government initiatives, and strategic business models are driving the revenue generation in the marketspace. In addition, North America is also observing the highest number of clinical trials for iPSCs. Currently, there are more than 58 clinical trials that are underway in this region. Moreover, institutes like NIH, California Institute for Regenerative Medicine (CIRM), National Institute of Neurological Disorders and Stroke (NINDS), and many more support induced pluripotent stem cells (iPSCs) related research activities.
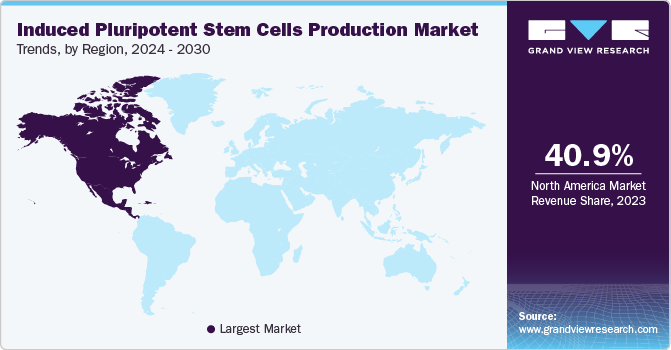
Asia Pacific is expected to grow at the fastest CAGR of 10.8% during the forecast period. Key factors such as intensive low-cost driven stem cell-based research, growing economies, betterment in healthcare plans, and the rising awareness of the potential of personalized treatments will drive revenue generation during the forecasted period. Moreover, the presence of multiple start-ups, academic universities, collaborative efforts by international companies, and new product/service launches in this region will contribute to APAC’s growth.
In June 2023, REPROCELL, Inc., a Japanese company, announced the debut of Pharmacology-AI, a brand-new paid service. The Hartree National Centre for Digital Innovation (HNCDI), in collaboration with IBM and the Science and Technology Facilities Council (STFC) Hartree Centre, has just finished one of the first EXCELERATE projects. REPROCELL and HNCDI worked together to develop EXCELERATE, a machine learning (ML) platform which streamlines and expedites the processing of large amounts of information from drug creation studies. Also, REPROCELL, Inc. and JTB Corp announced a business alliance in October 2022, and by using the Japan Medical & Health Tourism Centre (JMHC), a medical coordinating branch set up by JTB, they will start offering "Personal iPS" (REPROCELL's iPSC store as well as manufacturing service) worldwide.
Key Companies & Market Share Insights
In the induced pluripotent stem cells (iPSCs) production marketspace, key players are continuously expanding their product offerings by providing innovative & breakthrough technologies. Significant investments into R&D to provide disruptive products, business collaborations to expand the customer base, and strategic initiatives like partnership & acquisition to maintain market presence are driving the competition as well as fueling the growth dynamics.For instance, in January 2022, Bristol Myers Squibb and Century Therapeutics signed a strategic partnership agreement to work together to create allogenic cell treatments derived from iPSCs for the treatment of acute myeloid leukemia and multiple myeloma. Furthermore, in May 2022, Evotec and Sernova formed a strategic alliance to create an iPSC-based beta replacement therapy for the treatment of type 1 and type 2 diabetes.
Similarly, in July 2021, SCM Lifescience Co. Ltd., a South Korea based cell therapy developing company announced that it has in-license a diabetes drug from the US-based Allele Biotechnology and Pharmaceuticals, Inc. The deal is valued at USD 0.75 million, and the diabetes drug is a pancreatic beta cell therapy derived from iPSCs. Some of the prominent players in the global induced pluripotent stem cells production market include:
-
Lonza
-
Axol Bioscience Ltd.
-
Evotec
-
Hitachi, Ltd.
-
REPROCELL Inc.
-
Merck KGaA
-
REPROCELLS, Inc.
-
Fate Therapeutics
-
Thermo Fisher Scientific, Inc.
-
StemCellsFactory III
-
Applied StemCells, Inc.
Induced Pluripotent Stem Cells Production Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 1.48 billion
Revenue forecast in 2030
USD 2.76 billion
Growth rate
CAGR of 9.3% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Process, workflow, product, application, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Key companies profiled
Lonza; Axol Biosciences Ltd.; Evotec SE; Hitachi Ltd.; Merck KGaA; REPROCELLS, Inc.; Fate Therapeutics; Thermo Fisher Scientific, Inc.; StemCellsFactory III; Applied StemCells, Inc.
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Induced Pluripotent Stem Cells Production Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global induced pluripotent stem cells production market on the basis of process, workflow, product, product, end use, and region:
-
Process Outlook (Revenue, USD Million, 2018 - 2030)
-
Manual iPSC Production Process
-
Automated iPSC Production Process
-
-
Workflow Outlook (Revenue, USD Million, 2018 - 2030)
-
Reprogramming
-
Cell Culture
-
Cell Characterization / Analysis
-
Engineering
-
Others
-
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Instruments/ Devices
-
Automated Platforms
-
Consumables & Kits
-
Media
-
Kits
-
Others
-
-
Services
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Drug Development & Discovery
-
Regenerative Medicine
-
Toxicology Studies
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Research & Academic Institutes
-
Biotechnology & Pharmaceutical Companies
-
Hospitals & Clinics
-
-
Regional Outlook (Revenue in USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global induced pluripotent stem cells production market size was estimated at USD 1.37 billion in 2022 and is expected to reach USD 1.48 billion in 2023.
b. The global induced pluripotent stem cells production market is expected to grow at a compound annual growth rate of 9.30% from 2023 to 2030 to reach USD 2.8 billion by 2030.
b. The manual iPSCs production process segment dominated the global iPSCs production market and held the largest revenue share of 77.17% in 2022. It serves as a convenient method for parallel production of various iPSC lines, which has led to the segment’s dominance
b. Some key players operating in the iPSCs generation market include Lonza; Axol Biosciences Ltd.; Evotec; Hitachi Ltd.; Merck KGaA; REPROCELL Inc.; Fate Therapeutics; Thermo Fisher Scientific, Inc.; StemCellFactory III; and Applied StemCell Inc.
b. Key factors that are driving the iPSCs production market growth include a robust pipeline for induced pluripotent stem cell-derived cell therapeutics coupled with increasing demand for iPSCs.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





