- Home
- »
- Medical Devices
- »
-
Intracranial Pressure Monitoring Devices Market Size ReportGVR Report cover
![Intracranial Pressure Monitoring Devices Market Size, Share & Trends Report]()
Intracranial Pressure Monitoring Devices Market Size, Share & Trends Analysis Report By Technique (Invasive, Non-invasive), By Application (Traumatic Brain Injury, Intracerebral Hemorrhage), By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-1-68038-341-6
- Number of Pages: 110
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
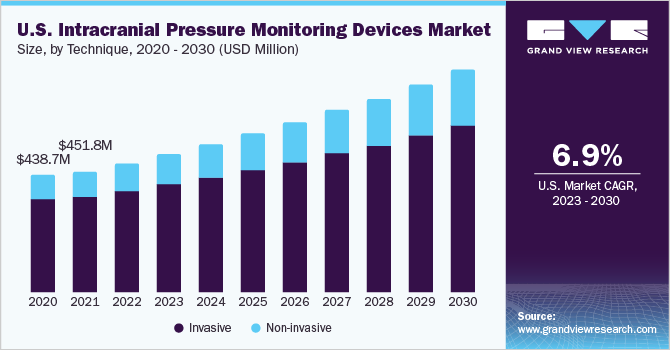
The global intracranial pressure monitoring devices market size was valued at USD 1.56 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 7.8% from 2023 to 2030. The growth of the market is attributed to the increasing prevalence of neurological disorders, the rapidly growing geriatric population in Asia Pacific, especially in countries such as Japan, India, and China, and rapid technological improvements. The COVID-19 pandemic is expected to have a short-term and moderate impact on the intracranial pressure (ICP) monitoring devices market. The COVID-19 pandemic has mostly provided an opportunity to minimize ICU utilization and accelerate patient release without increasing complications, readmissions, or reoperations.
In addition, stricter patient education, recovery room evaluation for non-ICU hospitalization, faster mobilization, post-discharge communication, increased adoption of minimally invasive surgeries, TIVA anesthesia, and early post imaging are expected to contribute to market growth. Hence, the existing invasive method is the primary technique utilized for intracranial monitoring. However, the development and usage of non-invasive methods for intracranial pressure monitoring are estimated to provide lucrative opportunities to key market players. For instance, according to ClinicalTrials.gov, as updated in April 2021, a clinical trial studying ‘intracranial pressure monitoring device performed in non-invasive method’ developed by Brain4Care is currently ongoing. This technology allows non-invasive assessment of the patient's status, which decreases a major risk of adverse effects while also lowering the expense of monitoring, which is expected to boost the growth of the market for intracranial pressure monitoring devices in near future.
In addition, a non-invasive intracranial pressure monitor may also be a significant research tool for studying pathophysiology and evaluating the efficiency of intracranial hypertension therapy. There might be prospects for space medical applications or studies on intracranial pressure responses at high altitudes. Hence, owing to the aforementioned factors, non-invasive intracranial pressure monitoring is expected to contribute to market growth, providing profitable opportunities for key players post COVID-19. A high-impact primary driver of the market for intracranial pressure monitoring devices is the growing need for minimally invasive surgical techniques due to improved patient outcomes.
In addition, the currently used ICP measurement requires puncturing a hole in the skull and inserting a catheter into the ventricular space for draining cerebrospinal fluid. There is a scarcity of experienced specialists, hence invasive procedures are not possible in a variety of situations, including emergencies. Thus, non-invasive methods such as transcranial Doppler ultrasonography, MRI/CT, and optic nerve sheath diameter are considered promising techniques to overcome these problems. For instance, Vittamed 205 developed by Vittamed Corporation, and Cerepress developed by Third Eye Diagnostics, are designed to measure and monitor ICP noninvasively via the eye.
In December 2022, Natus Medical Incorporated reached an agreement to acquire Micromed Holding SAS. Micromed and Natus work together to provide their customers with better neuromonitoring and neurodiagnostic solutions.
Similarly, HS-1000M Monitor by HeadSense Medical Ltd. and MMS-14 developed by Marchbanks Measurement Systems, use acoustic sound to measure and monitor ICP noninvasively through the ears. They are intended to be used in patients affected with stroke, traumatic brain injury/concussions, hydrocephalus, neurologic conditions, and other pathologies that may lead to intracranial hyper- and hypotension. Various benefits associated with these procedures, such as smaller incisions, faster recovery time, lower trauma, and low risk of complications, are contributing to market growth. Moreover, the increasing prevalence of neurological disorders, such as brain infection, hydrocephalus, aneurysm, intracranial tumors, and meningitis, and the growing incidence of trauma events due to road accidents, sports injuries, and falls worldwide are anticipated to drive the market for intracranial pressure monitoring devices.
For instance, according to the WHO, around 1.3 million people died globally in 2021 due to road accidents. In addition, between 20 and 50 million more people suffer non-fatal injuries, with many incurring a disability because of their injury. Road accidents are more common in middle- or low-income countries. Similarly, as per the same report, during 2009-2018, an estimated 596,972 ED visits for bicycle-related TBIs occurred in the U.S. These injuries or traumas result in elevated ICP, which in turn is expected to increase the demand for ICP monitoring devices, thereby positively influencing the growth of the market for intracranial pressure monitoring devices. In addition, the development of advanced devices, such as surgical microscopes, neurosurgery devices, ophthalmic surgical devices, and surgical robots, has led to a reduction in direct human interference in surgical procedures.
Technologically advanced ICP monitoring devices are reducing drawbacks of earlier invasive devices, such as a spontaneous shift in baseline pressure and leveling and de-bubbling associated with fluid-filled systems. For instance, medical robotics has been witnessing a paradigm shift over the years due to technological advancements in the area of 3D imaging. High-definition microscope cameras, data recorders, data analytic systems, motion sensors, remote navigation systems, and robotic-controlled catheters and transducers are some of the recent innovations. The aim of technological advancements is to find new applications for existing platforms as well as create disruptive technologies that drive the market for intracranial pressure monitoring devices.
The industry is witnessing a growing trend of robotic companies collaborating with third-party vendors to develop new applications of technology platforms. Furthermore, there is a trend of consolidation of technologies by big players in niche areas through mergers and acquisitions which is also increasing market growth. The U.S dominated the market with the highest share. This can be attributed to the increasing incidence of Traumatic Brain Injury (TBI) and migraine in the U.S. On the other hand, companies are also working on a range of strategic sourcing and diversity programs. For instance, in July 2019, Integra LifeSciences acquired Arkis BioSciences, Inc. This acquisition was expected to help Integra LifeSciences strengthen its product portfolio in neurocritical care and advanced catheter technology. Both companies will work on a range of areas, including co-marketing opportunities, product development, sustainable manufacturing, strategic sourcing, and diversity programs, which are expected to boost market growth soon.
Technique Insights
The invasive techniques segment dominated the market for intracranial pressure monitoring devices and held the largest revenue share of 79.6% in 2022. This is due to the fact that invasive ICP measurement can be performed at various intracranial anatomical locations (i.e., intraventricular, intraparenchymal, epidural, subdural, and subarachnoidal). Besides, according to the Department of Clinical Neurosciences, an intraventricular catheter coupled to an external pressure transducer is the gold standard for continuous ICP monitoring. This method has been shown to be the most accurate and low-cost method available for ICP monitoring, and it can also be used for therapeutic cerebrospinal fluid (CSF) drainage and administration of drugs. The two types of invasive techniques that are currently being used are external ventricular drain and micro-transducer ICP monitoring.
Over the projection period, the non-invasive segment is expected to witness the highest CAGR of 9.2%. This is due to invasive techniques associated with various complications such as infection, hemorrhage, malfunction, obstruction, and mispositioning of the catheter. Alternative noninvasive procedures for measuring elevated ICP are being sought due to the intrusive nature, high expense, and other extra dangers of current ICP monitoring methods. Because of a scarcity of experienced specialists, invasive treatments are not viable in a variety of situations, such as emergencies. Noninvasive treatments, including optic nerve sheath diameter, transcranial doppler ultrasonography, and magnetic resonance imaging/computed tomography (MRI/CT), are observed to be promising in overcoming these challenges.
Application Insights
In 2022, the traumatic brain injury segment dominated the market for intracranial pressure monitoring devices and accounted for a revenue share of around 31.5%. Elevated ICP is the most common cause of mortality in patients suffering from traumatic brain injury. The success of treatment is largely based on the accurate assessment of ICP. Besides, technological advancements are anticipated to propel market growth in the coming years. For instance, in August 2020, IRRAS AB, with a broad portfolio of innovative products for neurocritical care, has announced the first patient treatment with Hummingbird Solo, a new line extension to its innovative Hummingbird ICP Monitoring product family, which was launched in December 2019.
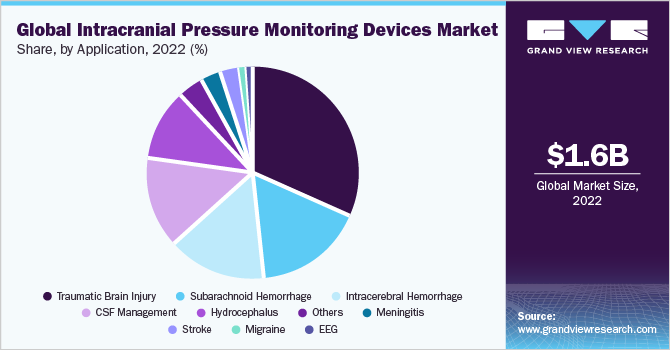
Hummingbird Solo is a single-lumen, bolt-based monitor that measures the pressure within a patient's brain tissue, manages ICP after traumatic brain injury, and aids in the diagnosis of subarachnoid hemorrhage or stroke. This is expected to increase its usage in diagnosing traumatic brain injury and other applications. The migraine segment is expected to witness the highest CAGR of 8.8% during the forecast period. This is due to the strong association between elevated ICP and obesity, it can be anticipated that increasing obesity in the general population will increase the prevalence and socioeconomic disease burden. According to the Journal of Headache and Pain in October 2018, idiopathic intracranial hypertension is a condition that primarily affects obese women of childbearing age. It is expected to affect 0.5 to 2 per 100,000 people of the general population which is anticipated to spur market growth.
Regional Insights
North America dominated the intracranial pressure monitoring devices market and accounted for the highest revenue share of 37.5% in 2022. Key factors expected to contribute to market growth in this region include the increasing availability of technologically advanced devices, growing demand for minimally invasive surgeries, and rising adoption of noninvasive ICP monitoring procedures. The market for intracranial pressure monitoring devices is also expected to be driven by an increasing number of trauma cases, as well as the rising incidence of migraine, stroke, and other neurological disorders. For instance, according to a report published by the American Association for the Surgery of Trauma in 2020, TBI is a leading cause of death and disability among children and young adults in the U.S. Each year, an estimated 1 million U.S. individuals suffer from TBI, among which 230,000 are hospitalized, 50,000 die, and 80,000 to 90,000 suffer from long-term disability. In addition, supportive government initiatives, including the establishment of the American Society of Craniofacial Surgery (ASCFS), for creating awareness about minimally invasive Craniomaxillofacial (CMF) surgeries are expected to further propel the market during the forecast period.
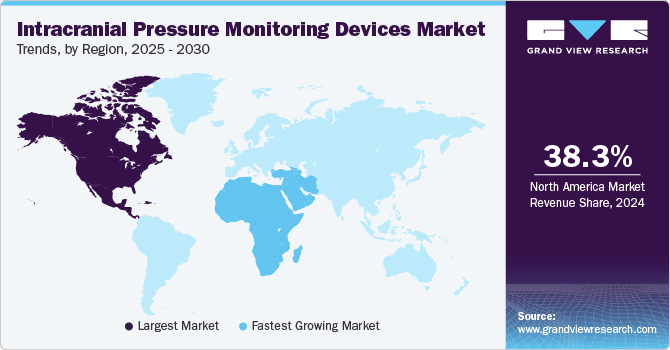
In Asia Pacific, the market for intracranial pressure monitoring devices is expected to witness a CAGR of 8.70% over the forecast period. The market is primarily driven by the increasing incidence of sports injuries and trauma. Emerging economies, such as China, Japan, South Korea, and India, are expected to witness considerable market growth over the forecast period. For instance, as per the CDC, people aged 65 years and above are at a high risk of falling, which can cause mild or severe brain injury. In addition, as per a WHO report, fall-related injuries in the elderly are a major health and social concern in China; approximately 18% to 44% of elderly persons are reported to fall each year. This is expected to present significant growth opportunities for the market over the coming years.
Moreover, the economy plays an important role in driving the market for intracranial pressure monitoring devices. In recent years, China has proven to be an attractive market for medical devices. In March 2019, the Chinese government reduced the Value Added Tax (VAT) for medical manufacturing companies from 13.3% to 13.0%. This was likely to benefit domestic manufacturers of medical equipment to offset the slowdown caused due to US-China trade war. Several companies, local and international, are trying to capture this opportunity by launching new and affordable products in the market. Such strategic initiatives undertaken by various companies to expand their businesses in untapped markets have led to several business transactions in the country, thereby contributing to market growth.
Key Companies & Market Share Insights
With the increased demand for intracranial pressure monitoring products, global manufacturers are speeding up manufacturing while also improving it with cost-effective solutions. For instance, in February 2023, NovaSignal Corporation announced that it has donated the NovaGuide 2 Intelligent Ultrasound to the Jacobs Institute. The Jacobs Institute continues research on the use of transcranial Doppler (TCD) in continuous neuromonitoring to improve neurovascular procedures and minimize the risk of stroke. Such developments are likely to offer neurosurgeons more insights into therapy and enable patients suffering from hydrocephalus patients to lead a more active life. Some of the prominent players in the global intracranial pressure monitoring devices market include:
-
Medtronic
-
Integra Lifesciences
-
Raumedic AG
-
Sophysa
-
Spiegelberg GmbH
-
Natus Medical
-
Gaelic Devices
-
Neural Analytics
Intracranial Pressure Monitoring Devices Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 1.68 billion
Revenue forecast in 2030
USD 2.85 billion
Growth rate
CAGR of 7.8% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
May 2023
Quantitative units
Revenue in USD million, and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Technique, application, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Medtronic; Integra Lifesciences; Raumedic AG; Sophysa; Spiegelberg GmbH; Natus Medical; Gaeltec Devices; Neural Analytics
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional, and segment scope
Pricing and Purchase Options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Intracranial Pressure Monitoring Devices Market Report Segmentation
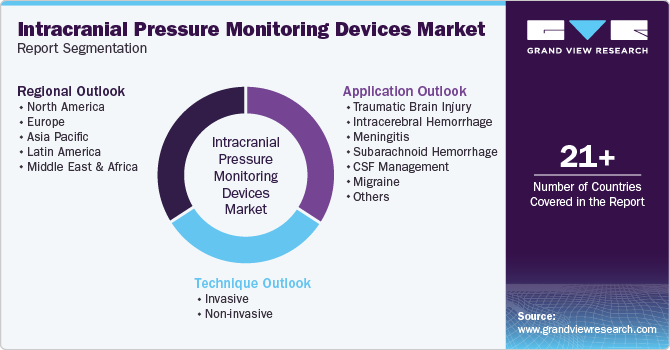
This report forecasts revenue growth at global, regional, & country levels and provides an analysis on the latest trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global intracranial pressure monitoring devices market report on the basis of technique, application, and region:
-
Technique Outlook (Revenue, USD Million, 2018 - 2030)
-
Invasive
-
External Ventricular Drainage (EVD)
-
Microtransducer ICP Monitoring
-
-
Non-invasive
-
Transcranial Doppler Ultrasonography
-
Tympanic Membrane Displacement (TMD)
-
Optic Nerve Sheath Diameter
-
MRI/CT
-
Fundoscopy (Papilledema)
-
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Traumatic Brain Injury
-
Intracerebral Hemorrhage
-
Meningitis
-
Subarachnoid Hemorrhage
-
CSF Management
-
Migraine
-
Stroke
-
Hydrocephalus
-
EEG
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global intracranial pressure monitoring devices market size was estimated at USD 1.56 billion in 2022 and is expected to reach USD 1.68 billion in 2023.
b. The global intracranial pressure monitoring devices market is expected to grow at a compound annual growth rate of 7.8% from 2023 to 2030 to reach USD 2.85 billion by 2030.
b. North America dominated the intracranial pressure monitoring devices market with a share of 37.48% in 2022. This is attributable to the increasing volume of intracranial pressure monitoring procedures, the rise in the number of trauma cases, and improved healthcare facilities.
b. Some of the key players operating in the intracranial pressure monitoring devices market include Medtronic, Codman & Shurtleff Inc, Raumedic AG, VIttamed, Sphysa Ltd., Orsan Medical Technologies, Integra LifeSciences Holding Corporation, and Spiegelberg GmbH & Co. KG.
b. Key factors that are driving the intracranial pressure monitoring devices market growth include the increasing prevalence of neurological disorders, rising demand for minimally invasive surgeries, the introduction of innovative devices, and increasing demand in trauma care.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





