- Home
- »
- Clinical Diagnostics
- »
-
Liquid Biopsy Market Size, Share & Trends Report, 2030GVR Report cover
![Liquid Biopsy Market Size, Share & Trends Report]()
Liquid Biopsy Market Size, Share & Trends Analysis Report By Biomarker (Exosomes, CTC), By Application (Cancer, Reproductive Health), By Technology, By Sample Type, By Clinical Application, By End-use, By Product, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-1-68038-105-4
- Number of Pages: 150
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Liquid Biopsy Market Size & Trends
The global liquid biopsy market size was estimated at USD 10.42 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 11.60% from 2024 to 2030. The market for liquid biopsy is witnessing growth due to factors such as the growing prevalence of cancer, technological advancements in cancer diagnostics, and rising preference for minimally invasive cancer diagnostics. Moreover, ongoing research for liquid biopsy assays and tests, aided with the rising adoption and development of multi-cancer early detection tests, provides opportunity for growth of overall market.
With the onset of COVID-19 pandemic, cancer diagnoses were delayed due to reduced diagnostic services and screening programs. Cancer patients are facing many challenges amid the pandemic, such as susceptibility to severe infection and interruption of cancer or usual medical care. The negative impact is likely to be stronger in low- and middle-income countries with poor infrastructure, limited resources, scarcity of medical supplies & personal protective equipment, and shortage of healthcare providers & organized care teams, resulting in a lack of ability to provide & deliver critical care.
For various applications, such as breast, colorectal, ovarian cancer, non-small-cell lung cancer, and prostate cancer, liquid biopsy is used for diagnostics & screening, making it a vital tool. After various studies and speculations, it has been derived that liquid biopsy technique could provide an improved diagnostic outcome. Data suggests the use of screening techniques on high-risk patients who have an ancestral history of cancer. Moreover, over the past several years, studies have shown positive outcomes of liquid biopsy platforms. The government and various regulatory bodies have also shown interest in the area by promoting multiple breakthrough devices for rapid development of the technology.
However, amid the pandemic, several companies have adopted various strategic initiatives for providing safe & easy in-home access for liquid biopsy tests. For instance, in November 2020, NeoGenomics, Inc. announced the launch of a mobile phlebotomy service for liquid biopsy tests, including InvisionFirst and NeoLAB. The company offers its service through two phlebotomy companies, Metro Health Staffing LLC and ExamOne, for broad geographic coverage to ensure tests are performed efficiently.
Market Dynamics
Liquid biopsy is an advanced testing technology for detection of genetic alteration-related tumors. It has also been utilized to stratify tumors and deliver precise cancer treatment. For instance, in January 2023, Guardant Health received FDA approval for Guardant360 CDx, a liquid biopsy assay as a companion diagnostics for ESR1 mutant breast cancer diagnosis. These recent innovations, advancements, and expansions in the industry promote the use of liquid biopsy and drive the market growth.
Furthermore, Multi Cancer Early Detection (MCED) provides crucial opportunities for growth of liquid biopsy industry. The MCED market represents an upcoming field of interest for the diagnostics industry, which not only allows early cancer detection but also facilitates early treatment of these patients by saving time and minimizing the risk of requiring invasive medical surgical procedures. There are many limitations to single cancer tests, such as high false-positive rates and optimized sensitivity. In addition, diagnosis is usually conducted with a focus on just one type of cancer thus, other cancers are left undiagnosed., Consequently, these single cancer detection tests are failing to meet the changing needs of the consumers.
Liquid biopsies are progressively studied as a tool that can determine tumor evolution and guide systemic treatment. According to a study by ASCO, it is insufficient to prove the clinical utility for most of the ctDNAs in advanced-stage cancers and early-stage disease screening. For ctDNA and CTC, there is a requirement to regulate pre-analytical variables for cross-platform comparison studies. However, to address these challenges, initiatives are being undertaken in the U.S. and Europe. Low concentration of ctDNA and CTCs in biological samples is expected to limit the usage of liquid biopsy in early-stage cancer diagnosis. Moreover, increasing ctDNA or CTC volume by drawing a large amount of blood from the patients is not clinically advisable. Such challenges in early-stage diagnosis are expected to restrain growth to a certain extent.
Technology Insights
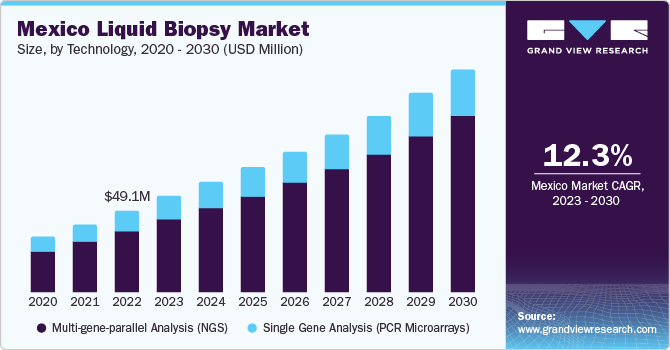
The multi-gene-parallel analysis (NGS) segment held the largest market revenue share of 75.68% in 2023 and is anticipated to grow at the fastest CAGR over the forecast period. NGS technology allows detection of various tumor-causing mutations and identification of potential emergence of post-treatment resistance mechanisms from pre-existing clones. Rapid developments in NGS technology have led to significant cost reductions in sequencing with high accuracy. Furthermore, key players operating in the market are developing innovative products to meet growing demand for diagnosis and maintain their position with an expanded product portfolio, thus driving the market growth. For instance, in January 2023, Agilent Technologies collaborated with Quest Diagnostics to provide access to Agilent Resolution ctDx FIRST, an NGS liquid biopsy test in the U.S.
Single Gene Analysis (PCR Microarrays) segment is anticipated to show significant growth during the forecast period. Technological advancements in PCR are expected to propel market growth over the forecast period. The recently introduced Droplet Digital PCR (ddPCR) is an advanced technology that allows absolute quantification of nucleic acids with high sensitivity and precision. This PCR technique has been developed as a rapid & precise tool for detecting and monitoring several types of cancers.For instance, Bio-Rad’s ddPCR technology detects cancer subtypes, monitors residual disease, optimizes drug treatment plans, and studies tumor evolution. ddPCR assays have advantages when used in liquid biopsies, enabling measurement of Circulating Tumor Cells (CTCs) and Circulating Nucleic Acids (cfDNA) in blood samples.
Biomarker Insights
Circulating Nucleic Acids biomarker segment held the largest market share of 35.96% in 2023,attributable to widespread applications associated with ctDNA in liquid biopsy. Translational cancer researchers are identifying ctDNA from tumors using liquid biopsy. The discovery of ctDNA offers new opportunities in future liquid biopsy applications for cancer diagnosis by acting as a possible biomarker. ctDNA has been suggested as an alternative source in cancer patients for molecular profiling of tumor DNA, as opposed to invasive techniques. A new technique for early detection and monitoring of cancer has been made possible by the aberrant ctDNA identification from cancer cells.
Exosomes/Microvesicles segment is anticipated to grow at the fastest CAGR over the forecast period.Exosomes show significant advantages in liquid biopsy. Exosomes are present in almost all body fluids, including plasma, cerebrospinal fluid, and urine. They possess high stability and are encapsulated by lipid bilayers. Exosomes act as a common central participant between cells during cancer progression and metastasis. The complex signaling pathway network between exosome-mediated cancer cells and the tumor microenvironment acts as a crucial factor in the cancer progression at all stages.
Application Insights
Cancer application segment dominated the overall market with a revenue share of 86.26% in 2023, owing to rising adoption of liquid biopsy in detection of cancer aided by the rising prevalence of cancer globally. Liquid biopsy technology is one of the most evolving technologies in diagnostics and has made considerable headway in recent years, showing a significant growth in adoption in clinical applications. This approach is a fast-emerging precision oncology tool that allows for longitudinal monitoring and less invasive molecular diagnostics for therapy purposes. Furthermore, in June 2022, Elypta raised USD 21 million for development of MCED test with LEVANTIS-0087A study in progress for MCED.
Reproductive health segment is anticipated to witness the fastest CAGR of 12.81% over the forecast period owing to promising R&D in liquid biopsy for treating and maintaining reproductive health. The reproductive application segment is expected to grow profitably throughout the projection period. Furthermore, alliances and partnerships among reproductive health industry actors promote segment expansion. For instance, in September 2021, Bionano Genomics and NuProbe entered into a partnership on reproductive health and oncology liquid biopsy testing to give an option to discover variations that NGS cannot detect.
End-use Insights
The hospitals and laboratories end-use segment dominated the market in 2023 with a revenue share of 42.33% in 2023. Hospitals are preferred for care due to availability of various services under one roof. The benefit of hospitals conducting cancer diagnosis is that they can offer results for tests even in emergencies. Liquid biopsy is helping doctors by giving them a highly precise cancer diagnosis in a shorter turnaround time, thereby reducing the treatment lag time. Cancer patients in hospitals undergo routine monitoring for analysis of resistance to treatment. Chemotherapy has long been a successful and dependable cancer treatment. Chemotherapy may be used to treat cancer or to improve quality of life by symptom management. In addition, chemotherapy can improve the efficacy of other treatments like surgery or radiation therapy.
Specialty clinics segment is anticipated to grow at the fastest CAGR of 12.22% over the forecast period. An increase in awareness of personalized medicine, technological advancements, and a rise in demand for affordable services are some of the key factors expected to drive growth of specialty clinics segment. Another major factor expected to drive market growth is increasing government initiatives to provide various facilities, such as compensation for diagnostic tests. Moreover, a number of healthcare institutions are working with laboratories to incorporate different clinical tests, such as microbiology testing.
Clinical Application Insights
Therapy selection segment dominated the overall market in 2023 with a revenue share of 33.76%. The market's growth is attributed to selection of treatment options that can be affected by liquid biopsies to improve patient outcomes. Cancer is the second leading cause of mortality in the U.S. and most expensive disease to cure. Several advancements in cancer detection and biomarkers have been developed to aid the study of cancer progression and creation of successful treatment options. Liquid biopsies can assist in improving cancer therapies by enabling early intervention, enhancing treatment control, and moving decision-making away from reactionary acts and toward more proactive early interventions. Early detection is also facilitating market growth.
Early screening segment is expected to show the fastest CAGR of 12.44% over the forecast period. The segment growth is attributed to rise in prevalence of multiple cancers and the increasing need to provide efficient methods to detect them at early stages to enable timely appropriate treatment, are anticipated to drive the market growth. For instance, according to the International Agency for Research on Cancer (IARC) over 20.7 million new cancer cases are expected in 2023. The need to develop diagnostic options that can detect cancer at an early stage, which can help improve disease management & reduce mortality, is likely to propel the overall market.
Product Insights
The instruments product segment dominated the market in 2023 with a revenue share of 46.47%, attributed to the launch of new instruments and the advancement of existing products. For instance, in 2022, Tempus added xF+, a liquid biopsy panel made of 523 genes, a brand-new non-invasive test that focuses on pathogenic mutations in cfDNA, to its array of comprehensive genomic profiling services.
Kits and reagents segment is expected to be the fastest-growing segment during the forecast period, owing to increasing research and development activities by key market players for development of advanced forms of kits and reagents. For instance, in 2021, Sysmex Inostics GmbH and Sysmex Europe GmbH launched the Plasma-SeqSensei liquid biopsy kits for thyroid, non-small cell lung cancer, colorectal cancer, and melanoma cancer, only for research use. The recurring cost associated with kits and reagents further propels the segment growth.
Sample Type Insights
Blood sample segment held the largest market share of 71.69% in 2023 and is projected to maintain its dominance over the forecast period. Blood-based liquid biopsy has remarkable advantages over traditional biopsy methods. Blood-based liquid biopsies are non-invasive, painless, and have no risk. In addition, it reduces time taken and cost for diagnosis. Exomes, CTCs, cfDNAs, and microvesicles in a blood sample can be detected, thus increasing adoption of blood-based liquid biopsy. Circulating biomarkers play a vital role in understanding tumorigenesis and metastasis, which help determine tumor dynamics for treatment and disease progression. Moreover, concentration of biomarkers in blood might allow quick detection of cancer stage and enable more favorable prediction regarding prognosis in patients.
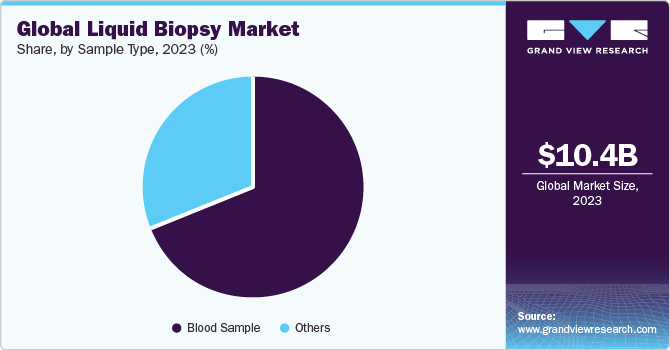
Others segment is anticipated to grow at the fastest CAGR of 12.75% over the forecast period.Others segment includes urine, saliva, and Cerebrospinal Fluid (CSF)-based tests. Urine has also been used extensively for urinalysis and has proven applications in medical diagnosis. While it is not as commonly used in liquid biopsy studies, a few companies focus on developing urine-based liquid biopsy products. Collection of urine samples in large quantities is non-invasive, easier, and inexpensive. For instance, in August 2021, Nonacus entered a partnership with the University of Birmingham to develop a non-invasive urine-based test for detection of bladder cancer.This test uses the company’s liquid biopsy technology and a panel of biomarkers approved by a team of scientists at the Bladder Cancer Research Centre of the University of Birmingham to diagnose the disease from a urine sample.
Regional Insights
North America dominated the market with a revenue share of 50.76% in 2023, owing to high cancer prevalence, rapid technological advancements, and growing government initiatives. Moreover, the market is led by the U.S. owing to more investments and presence of several biotechnology companies developing advanced tests. Various organizations, including the American Society of Clinical Oncology (ASCO), are working to support the deployment of liquid biopsy, which is expected to increase revenue in this market. The market is expected to grow during the forecast period owing to intense competition between biotechnology companies and increasing investments by governments in healthcare institutes to develop more sophisticated tests.
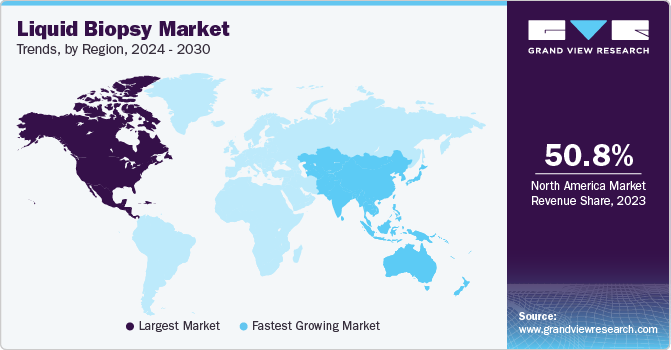
The market in Asia Pacific is expected to grow at the fastest CAGR of 13.09% over the forecast period due to various factors, such as improving healthcare reforms. Other factors contributing to market growth are increasing population, improving healthcare infrastructure, and entry of new players. Asia Pacific has a large population and a high prevalence of cancer. According to Global Cancer Statistics, estimated number of new cases of cancer in Asia in 2022 was 10.5 million. Government initiatives, such as free screening for breast, cervical, & lung cancer and improved collaborations between the government, research institutes, & companies for distribution & supply of these tests for screening cancers, have increased in the past few years.
Key Companies & Market Share Insights
The presence of pipeline products in the liquid biopsy segment that are expected to launch in the coming years is anticipated to drive the market growth over the forecast period.
-
In November 2023, Illumina Inc. announced the new TruSight Oncology 500 ctDNA v2, a new generation of its liquid biopsy assay for genomic profiling.
-
In January 2023, Agilent Technologies, Inc. acquired Avida Biomed. This initiative is expected to provide it with rapid growth in the diagnostics and clinical research markets.
Key Liquid Biopsy Companies:
- ANGLE plc
- Oncimmune Holdings PLC
- Guardant Health
- Myriad Genetics, Inc.
- Biocept, Inc.
- Lucence Health Inc.
- Freenome Holdings, Inc.
- F. Hoffmann-La Roche Ltd.
- QIAGEN
- Illumina, Inc.
- Thermo Fisher Scientific, Inc.
- Epigenomics AG
Liquid Biopsy Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 11.84 billion
Revenue forecast in 2030
USD 22.88 billion
Growth Rate
CAGR of 11.60% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2021
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Sample type, biomarker, technology, end-use, application, clinical application, product, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; U.K.; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
QIAGEN; Myriad Genetics, Inc.; Biocept, Inc.; Guardant Health; F. Hoffmann-La Roche Ltd; Illumina, Inc.; ANGLE plc; Oncimmune Holdings PLC; Thermo Fisher Scientific, Inc.; Lucence Health, Inc.; Freenome Holdings, Inc.; Epigenomics AG
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Liquid Biopsy Market Report Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the global liquid biopsy market report based on sample type, biomarker, technology, application, end-use, clinical application, product, and region:
-
Sample Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Blood Sample
-
Others
-
-
Biomarker Outlook (Revenue, USD Million, 2018 - 2030)
-
Circulating Tumor Cells (CTCs)
-
Circulating Nucleic Acids
-
Exosomes/ Microvesicles
-
Circulating Proteins
-
-
Technology Outlook (Revenue, USD Million, 2018 - 2030)
-
Multi-gene-parallel Analysis (NGS)
-
Single Gene Analysis (PCR Microarrays)
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Cancer
-
Reproductive Health
-
Lung Cancer
-
Prostate Cancer
-
Breast Cancer
-
Colorectal Cancer
-
Leukemia
-
Gastrointestinal Cancer
-
Others
-
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals and Laboratories
-
Specialty Clinics
-
Academic and Research Centers
-
Others
-
-
Clinical Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Therapy Selection
-
Treatment Monitoring
-
Early Cancer Screening
-
Recurrence Monitoring
-
Others
-
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Instruments
-
Consumables Kits and Reagents
-
Software and Services
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
U.K.
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global liquid biopsy market size was estimated at USD 10.42 billion in 2023 and is expected to reach USD 11.85 billion in 2024.
b. The global liquid biopsy market is expected to grow at a compound annual growth rate of 11.60% from 2024 to 2030 to reach USD 22.88 billion by 2030.
b. North America dominated the liquid biopsy market with a share of 50.76% in 2023. This is attributable to high cancer prevalence, rapid technological advancements, and growing government initiatives. Moreover, the market is led by U.S. owing to greater investments and presence of several biotechnology companies that are developing advanced tests.
b. Some key players operating in the liquid biopsy market include QIAGEN; Myriad Genetics, Inc; BIOCEPT, Inc; Guardant Health; F. Hoffmann-La Roche Ltd; Illumina, Inc; ANGLE plc; Oncimmune Holdings PLC; Thermo Fisher Scientific, Inc.; Lucence Health, Inc.; Freenome Holdings, Inc.; Epigenomics AG
b. Key factors that are driving the market growth include factor such as the growing prevalence of cancer, technological advancements in cancer diagnostics, and rising preference for minimally invasive cancer diagnostics. Moreover, ongoing research for the development of liquid biopsy assays and tests aided with the rising adoption and development of multi-cancer early detection tests is providing a major opportunity for growth of overall market.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





