- Home
- »
- Medical Devices
- »
-
Medical Writing Market Size, Share & Trends Report, 2023GVR Report cover
![Medical Writing Market Size, Share & Trends Report]()
Medical Writing Market Size, Share & Trends Analysis Report By Type (Clinical, Regulatory), By Application (Medical Journalism, Medico Marketing), By End Use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-2-68038-908-1
- Number of Pages: 145
- Format: Electronic (PDF)
- Historical Range: 2016 - 2021
- Industry: Healthcare
Report Overview
The global medical writing market size was valued at USD 3.8 billion in 2022 and is expected to expand at a CAGR of 10.46% from 2023 to 2030. The high demand for medical writers can be attributed to a rise in CRO outsourcing, increased R&D investments by market players, a favorable environment for clinical study in developing countries, and new medical device regulations. According to a study conducted in 2018, around 80% of internet users in the U.S. searched online for health-related information. In addition, nearly 60% of social media users rely on health information posted on social media platforms. Doctors can expand their reach for better patient care by engaging with patients on these platforms. Hence, the demand for online medical writing is increasing. However, the information posted on such forums should be by medical writing guidelines.
In recent years, pharmaceutical companies have adapted their advertising strategies to improve patient outreach. In addition, the growing influence of social media on customers’ decisions has compelled pharmaceutical and biotechnology companies to reconsider their marketing strategies. Hence, the robust growth of health and wellness-related blogs is expected to boost the demand for scientific writing.
Regulatory authorities want a thorough methodology for all phases of product development, which complicates the approval process boosting the market growth in the coming years. Additionally, insurance companies want drug information in order to establish reimbursement policies. As a result, pharmaceutical companies are finding it difficult to adhere to industry standards.
The pharmaceutical industry spends thrice the amount spent on R&D in other high-tech industries such as computer & software services. As per the Congressional Budget Office (CBO) report, R&D spending on pharmaceuticals reached USD 83 billion in the U.S. in 2019. Around 30 top pharmaceutical companies have collaborations with at least one CRO to maintain their product portfolios.
R&D activities provide market players with a competitive edge in the market. There were a total of 62,092 recruiting studies as of March 24, 2021, according to data given by ClinicalTrails.gov. Top pharmaceutical, medical devices, and biotechnology companies spend a significant share of their revenues on R&D activities to maintain their position in the market by introducing innovative products.
COVID-19 Medical Writing Market Impact: 0.5 % decrease from 2019 to 2020
Pandemic Impact
Post COVID-19 Outlook
The sudden onset of the pandemic halted R&D activities and clinical trials. In addition, many pharmaceutical companies cut their investments due to budget constraints. This hampered the market growth
The COVID-19 pandemic proved the value of companies and research groups collaborating with governments to develop novel therapies. As a result, medical device, biotechnology, and life science companies are planning to increase their spending on medical writers.
The market contracted by 0.5% year-on-year in 2020, compared to 2019. Most healthcare journals witnessed a decline in submissions attributed to the outset of the pandemic.
The growing number of clinical trials, especially for the COVID-19 vaccine and treatment is anticipated to surge the demand for medical writers. In the next years, this will provide a considerable opportunity for clinical writing service providers.
There is a new trend in the market of multiple strategic relationships, wherein each CRO provides a single function and multiple service providers are maintained. Clinical trials are increasing in number as a result of increased R&D investments. However, the number of clinical writers is not increasing at the same rate, resulting in the shortage of skilled writers in the field of medicine, which is expected to increase the scope for new writers in the field of medicine.Type Insights
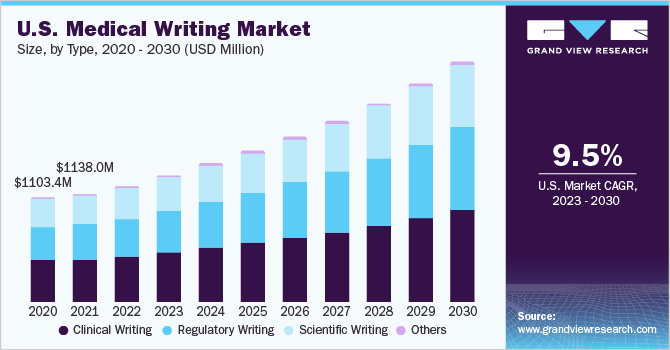
Based on type, the clinical writing segment dominated the global medical writing market with a share of 38.1% in 2022. The segment is estimated to dominate the market throughout the forecast period. Clinical writing is used by health professionals on a regular basis and a clinical writer must have a thorough knowledge of the clinical language as well as culture. An understanding of the target reader and the purpose is required for effective clinical writing.
It is used in protocols, consent documents, and clinical study reports for all the phases of clinical trials in product development. It mainly focuses on professionals with a sound comprehension of drug/product development processes and analysis of scientific data.
Regulatory writing is expected to be the fastest-growing segment with a CAGR of 11.30% during the forecast period. It is used throughout the process of product development for clinical documentation. It is required for describing and reporting data from clinical trials for the purpose of regulatory submissions and approvals. When the product or drug receives approval, post-approval regulatory documentation is required. Thus, stringent regulations are resulting in increasing documentation which is boosting the segment growth.
Application Insights
The medical journalism segment accounted for the maximum share of the market with 38.5% in 2022. The segment is mainly concerned with investigating, reporting, and communicating health issues to a wide audience. This type of journalism targets nonprofessional/non-expert audiences, along with healthcare professionals. Based on application, the global medical writing market is segmented into medical journalism, medical education, medico marketing, and others.
Health-related issues are of common interest to most people, as they have an impact on the behavior of people. Rapid advancements in the field of medicine and the growing importance of health information have increased the need for writers who are capable of comprehending and communicating health-related information through their writing.
On the other hand, the medico marketing segment is expected to witness the highest CAGR during the forecast period. Medico-marketing involves designing marketing content for drugs and other products. The ever-changing product mix in the pharmaceutical & biotechnology industry acts as a driving factor for improved marketing practices.
End-Use Insights
Based on end-use, the CROs & other segments dominated the market in 2022 due to rising R&D costs. The segment includes medical device/pharmaceutical & biotechnology companies, and CROs & others. Drug manufacturers are under pressure to replace revenue loss specifically due to the introduction of generics post-patent expiration.
CROs conduct clinical trials to introduce new products in the market in given timelines. They have the required expertise and infrastructure that give the benefit of cost, time, and efficiency at the same time. CROs have a separate service section for medical writing. Emerging economies such as Japan, India, and China are preferred countries for outsourcing these activities. Many of these countries have given better offers to the experts in Western countries.
On the other hand, medical device, pharmaceutical & biotechnology companies hire writers or journalists frequently. Established pharmaceutical companies have a separate team of medical writers. In addition, various companies outsource writers.
Regional Insights
North America dominated the market with a share of 35.4% in 2022. Clinical writing in the U.S. for FDA approval requires an in-depth understanding of the requirements laid down by the regulatory authority. The documents for FDA submission must be succinct and accurate.
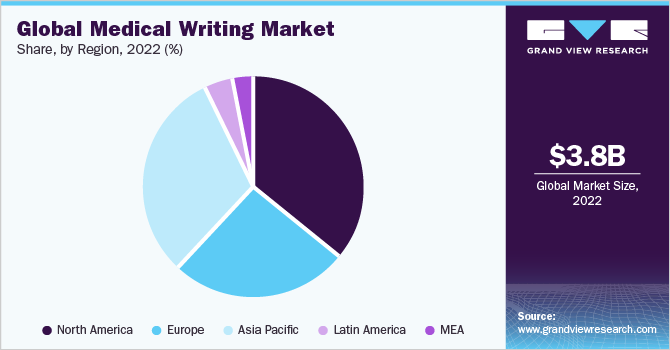
North America's growth is attributed to the existence of numerous pharmaceutical companies and considerable drug development operations in the US. However, numerous US pharmaceutical corporations use low-cost offshore countries for contract research organization (CRO) activities resulting in discrepancies between consumption and production.
On the other hand, Asia Pacific is likely to witness a lucrative growth rate over the forecast period from 2023 to 2030. The need for quality writing is increasing in the Asia Pacific as it is being recognized as an emerging profession. China and India are likely to dominate the regional market owing to inexpensive labor, quick turnaround times, an abundance of English writers, and easy availability of resources.
Key Companies & Market Share Insights
Top pharmaceutical, medical device, and biotechnology companies are significantly investing in R&D activities to maintain their position in the market by introducing innovative products. Research and development activities provide market players with a competitive advantage over their competitors.
Strategic alliances between large pharma companies and CROs have become an ongoing trend. For instance, the merger between Quintiles and IMS Health was the biggest one in the contract research organization (CRO) industry. Some of the prominent players in the medical writing market include:
-
Parexel International Corporation
-
Trilogy Writing & Consulting GmBH
-
Freyr
-
Cactus Communications
-
Labcorp Drug Development
-
IQVIA Holdings Inc.
-
Omics International
-
Synchrogenix
-
Siro Clinpharm Private Limited
-
Quanticate
-
Inclin, Inc.
Medical Writing Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 4.2 billion
Revenue forecast in 2030
USD 8.4 billion
Growth Rate
CAGR of 10.46% from 2023 to 2030
Base year for estimation
2022
Historical data
2016 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, application, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S., Canada, U.K., Germany, France, Italy, Spain,Norway, Sweden, Denmark Japan, China, India, Australia, South Korea, Thailand, Brazil, Mexico, Argentina, South Africa, UAE, Saudi Arabia, Kuwait
Key companies profiled
Parexel International Corporation; Trilogy Writing & Consulting GmBH; Freyr; Cactus Communications; Labcorp Drug Development; IQVIA Holdings Inc.; Omics International; Synchrogenix; Siro Clinpharm Private Limited; Quanticate; Inclin, Inc.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
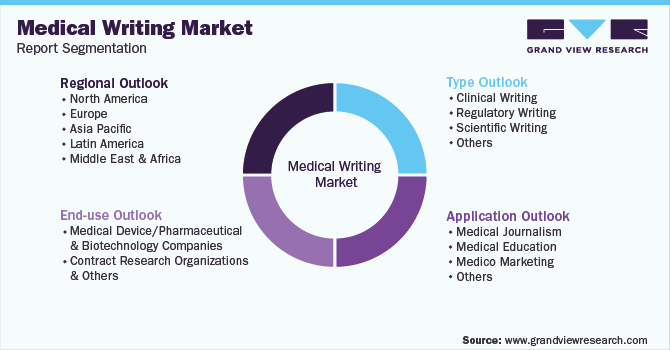
Global Medical Writing Market Report SegmentationThis report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2016 to 2030. For the purpose of this study, Grand View Research has segmented the global medical writing market report based on type, application, end-use, and region:
-
Type Outlook (Revenue, USD Million, 2016 - 2030)
-
Clinical Writing
-
Regulatory Writing
-
Scientific Writing
-
Others
-
-
Application Outlook (Revenue, USD Million, 2016 - 2030)
-
Medical Journalism
-
Medical Education
-
Medico Marketing
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2016 - 2030)
-
Medical Device/Pharmaceutical & Biotechnology Companies
-
Contract Research Organizations & Others
-
-
Regional Outlook (Revenue, USD Million, 2016 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Norway
-
Sweden
-
Denmark
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa (MEA)
-
South Africa
-
United Arab Emirates (UAE)
-
Saudi Arabia
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global medical writing market size was estimated at USD 3.8 billion in 2022 and is expected to reach USD 4.2 billion in 2023.
b. The global medical writing market is expected to grow at a compound annual growth rate of 10.46% from 2023 to 2030 to reach USD 8.4 billion by 2030.
b. North America dominated the medical writing market with a share of 35.4% in 2022. Medical writing in the U.S. for FDA approval requires an in-depth understanding of the requirements laid down by the regulatory authority. The documents for FDA submission must be succinct and accurate.
b. Some of the key players in the medical writing market are IQVIA; Parexel International Corp.; Trilogy Writing & Consulting GmBH; Labcorp Drug Development; OMICS International; Synchrogenix; Siro Clinpharm Private Limited; Quanticate; Inclin, Inc.; and Freyr.
b. High demand for medical writing services can be attributed to a rise in CRO outsourcing, increased R&D investments by market players, favorable environment for clinical trials in developing regions, new medical device regulations, and increasing penetration of internet and social media.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





