- Home
- »
- Clinical Diagnostics
- »
-
Molecular Diagnostics Market Size And Share Report, 2030GVR Report cover
![Molecular Diagnostics Market Size, Share & Trends Report]()
Molecular Diagnostics Market Size, Share & Trends Analysis Report By Product (Reagents, Instruments), By Technology (PCR, INAAT), By Application (Oncology, Genetic Testing), By Test Location, And Segment Forecasts, 2024 - 2030
- Report ID: 978-1-68038-086-6
- Number of Pages: 270
- Format: Electronic (PDF)
- Historical Range: 2018 - 2022
- Industry: Healthcare
Molecular Diagnostics Market Size & Trends
The global molecular diagnostics market size was estimated at USD 15.20 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 4.5% from 2024 to 2030. Factors, such as technological advancements, an increasing geriatric population, and increasing demand for accurate & effective genetic testing are boosting market growth. Moreover, rising demand for point-of-care testing can be attributed to increasing demand for self-testing diagnostics and rising patient awareness about faster diagnostics, which is encouraging industry players to develop novel testing products. For instance, in February 2023, Huwel Lifesciences designed a portable RT-PCR machine to test different types of viruses.
The company claimed that the test takes around 30 minutes and can be used to conduct the test for respiratory and other infections using blood and gastrointestinal samples. The rising geriatric population globally is at a higher risk of developing numerous diseases, including cancer, cardiovascular diseases, obesity, neurological disorders, and diabetes. According to the World Bank Group data, in 2022, there were about 779 million people aged 65 years & above globally. In addition, the number of individuals aged 80 years and above is projected to double by 2050, to surpass more than 1.5 billion. Thus, growing geriatric population is expected to be a high-impact rendering driver of the market. In addition, the growing incidence of infectious diseases is expected to propel market growth.
Rising target population due to high incidence of STIs, such as HIV and HPV, is expected to drive industry growth. According to the WHO, the prevalence and incidence of HIV were approximately 39 million and 1.3 million, respectively, in 2022 across the globe. Furthermore, people suffering from HIV are highly susceptible to other infections, such as tuberculosis, which is the leading cause of death among HIV-affected people. Rising number of initiatives by key players to improve access to cost-effective resources is anticipated to drive market growth. Molecular diagnostics render accurate & effective results and have indispensable applications in disease diagnostics. For instance, in December 2023, Co-Diagnostics, Inc. submitted documents for review of the Co-Dx PCR COVID-19 test with Co-Dx PCR Pro instrument to the U.S. FDA for emergency use authorization.
These tests and instruments are designed for use at POC and at-home settings. Increasing introduction of novel molecular diagnostic products and favorable initiatives undertaken by government and non-government bodies further support the market expansion. For instance, in November 2023, F. Hoffmann-La Roche Ltd launched LightCycler PRO System to raise the usability of molecular PCR testing in translational research and in vitro diagnostic applications. Similarly, BARDA’s 2022-2026 Strategic Plan supports development and authorization of advance pathogen-specific diagnostics. Moreover, BARDA supports T2 Biosystems’s T2Biothreat Panel, a blood molecular diagnostic test, which can detect six pathogens in a single sample.
Market Concentration & Characteristics
Market growth stage is medium, and pace of the market growth is accelerating. The market is characterized by a high degree of innovation owing to the increasing introduction of novel tests using PCR, NGS, and other molecular diagnostic techniques for different healthcare conditions. Moreover, key players are actively involved in the development of novel POC testing products to capture the market opportunities.
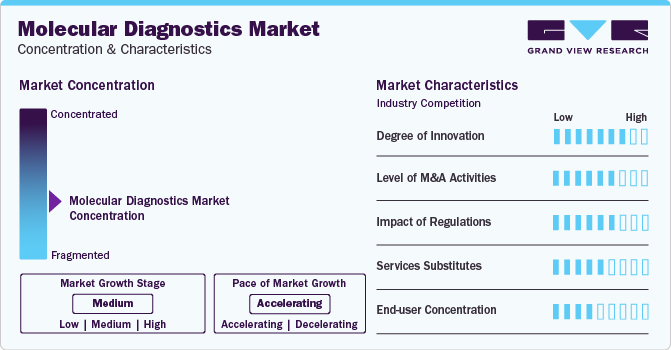
The market is also characterized by a high level of merger and acquisition (M&A) activity by the leading players. For instance, in April 2023, Quest Diagnostics Incorporated acquired MRD Platform of Haystack Oncology, a circulating tumor DNA-based technology for early detection of cancer and personalized medicine. Companies are adopting these strategies to increase their expertise and gain access to new regions in the rapidly growing market.
The market is subject to increasing regulatory scrutiny. The regulatory framework for diagnostic product approvals is one of the major factors restraining market growth. Moreover, the regulatory atmosphere is already complex, and the FDA is becoming more careful as dependency on molecular diagnostics for making serious medical decisions is growing. The FDA also conducts post-market investigations of diagnostic products to ensure parity between performances and claims.
The presence of substitute technologies, such as immunoassay and other microbiology testing, can hamper the market uptake of molecular diagnostic products. Moreover, the cost-effectiveness and increasing accuracy of immunoassay coupled with rapid test results make it a suitable substitute for molecular diagnostics.
End-user concentration is a major factor in the market. End-users, such as hospitals, clinics, laboratories, and POC facilities, are driving demand for MDx tests. Moreover, increasing demand for accurate, rapid, and self-tests for cancer, biological markers, genetic information for inherited conditions, and others opens new avenues for market players in different end-use applications.
Product Insights
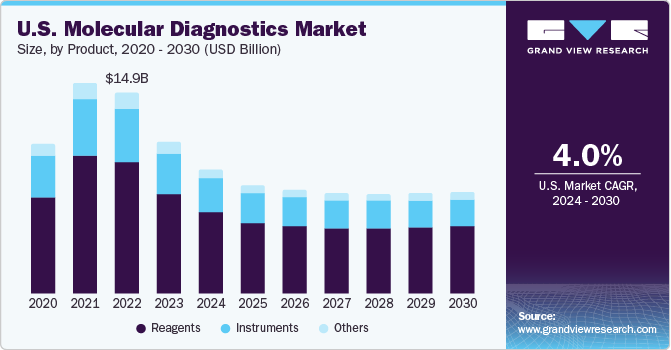
The reagents segment dominated the market and accounted for a share of 62.2% of the global revenue in 2023. It is expected to maintain its dominance throughout the forecast years owing to its wide application scope in research & clinical settings and increasing adoption of novel tests. For instance, in April 2023, Cepheid, a subsidiary of Danaher, announced a plan to introduce novel tests for a range of infectious diseases, including respiratory diseases and tuberculosis. The company has collaborated with researchers at Rutgers University to develop this novel technology that allow to detect 10 independent targets in one reaction. Moreover, standardized results, improved efficiency, and cost-effectiveness are anticipated to support market growth.
The instruments segment held the second-largest revenue share in 2023 due to the extensive use of instruments in different healthcare facilities to diagnose life-threatening diseases. Moreover, introduction of technologically advanced instruments is further supporting the segment growth. For instance, in February 2023, Thermo Fisher Scientific, Inc. announced the release of QuantStudio, Applied Biosystems, Absolute Q AutoRun dPCR Suite, a new digital PCR research tool. Moreover, in May 2023, Sansure Biotech presented its comprehensive range of IVD products, including the iPonatic III portable molecular workstation, at EuroMedLab Rome 2023.
Technology Insights
The polymerase chain reaction technology segment accounted for the largest revenue share in 2023. This is attributed to its use in detecting COVID-19 and other infectious diseases. The increasing use of high-throughput PCR technology to detect infectious diseases and genetic or inherited conditions is expected to drive market growth. For instance, in April 2023,Molbio Diagnostics launched Truenat H3N2/H1N1, the initial PoC Real-Time Polymerase Chain Reaction (PCR) test that assists in the proven identification of influenza infections. The sequencing technology segment is expected to grow at the fastest CAGR from 2024 to 2030.
The segment growth can be attributed to the rising penetration of technology in diagnostic applications, introduction of novel NGS tests, and favorable initiatives by key players. For instance, in April 2023, Alercell launched the LENA Molecular Dx Leukemia Platform, which comprises 12 molecular diagnostic tests based on next-gen DNA sequencing technology. Moreover, in January 2023, QIAGEN and Helix established an exclusive agreement to improve next-gen sequencing companion testing in genetic illnesses. DNA sequencing technologies are integrally linked to drug discovery, novel drug development, and personalized medicine. Companies are launching new NGS-based tests for early disease diagnosis.
Application Insights
The infectious diseases segment accounted for the largest revenue share in 2023. The increased usage of molecular, particularly PCR tests, for diagnosing COVID-19 has increased the segment share significantly. Moreover, the increasing prevalence of infectious diseases across the globe is further increasing the demand for novel molecular diagnostic tests. For instance, in May 2023, the U.S. FDA approved 510(k) clearance for Hologic's Panther Fusion SARS-CoV-2/Flu A/B/RSV assay. This assay is a molecular diagnostic test that discriminates between the four utmost common respiratory viruses. Moreover, in April 2023, Oxford Nanopore and bioMérieux formed a strategic partnership involving nanopore technology to develop novel treatments for infectious diseases.
The oncology segment is expected to grow at a high CAGR from 2024 to 2030. According to the American Cancer Society estimates, in 2023, about 288,300 new cases of prostate cancer and around 59,610 new cases of leukemia were estimated to be diagnosed in the U.S. Moreover, increasing collaboration activities between research institutes and market players to develop new diagnostic solutions for cancer is supporting the segment growth. For instance, in October 2023, QIAGEN and Myriad Genetics collaborated to develop companion diagnostic tests based on PCR, dPCR, and NGS technology for cancer.
Test Location Insights
The central laboratories segment dominated the industry in 2023 owing to high procedure volumes for COVID testing and other healthcare indications in central laboratories.In April 2023, ELITechGroup announced its plans to introduce a high throughput sample-to-result molecular diagnostics equipment to help laboratories worldwide. Moreover, a rise in the number of initiatives undertaken by governments to provide various services, such as reimbursement for diagnostic tests, is another major factor anticipated to drive market growth.
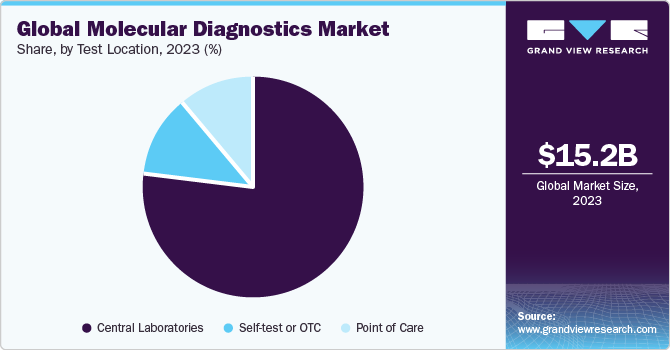
There is an increasing interest in the development of molecular diagnostic platforms that can also be used as OTC or in at-home settings. Therefore, various companies are designing assays & molecular diagnostic platforms that patients can use without the help of healthcare professionals. For instance, in March 2023, Lucira Health launched the first and only at-home COVID-19 and flu tests in the U.S. The U.S. FDA gave the COVID-19 & Flu Home Test the first and only Emergency Use Authorization (EUA) for OTC usage at home and other non-laboratory locations.
Regional Insights
North America dominated the market and accounted for a 39.3% share in 2023. This is attributed to the rising epidemiology of infectious as well as chronic diseases, thus, encouraging companies to introduce novel molecular diagnostic tests, thereby boosting market growth. For instance, in August 2023, QIAGEN received the U.S. FDA approval for therascreen PDGFRA RGQ PCR kit, this aids physicians in identifying patients with gastrointestinal stromal tumors (GIST). Moreover, in January 2023, the U.S. FDA approved EUA for the VIASURE Monkeypox Virus Real-Time PCR Reagents developed by BD and CerTest Biotec for Mpox virus detection. Moreover, high standards of living and consumer awareness about early diagnosis, as well as well-established healthcare system, act as key drivers for growth.
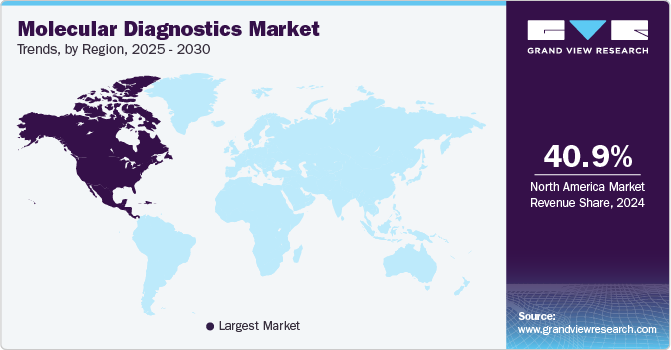
Asia Pacific is anticipated to exhibit significant growth from 2024 to 2030 owing to increased market penetration, initiatives of local market players to increase the adoption of novel diagnostic technologies, and high unmet market needs. For instance, in August 2023, Indian companies CrisprBits and MolBio Diagnostics entered into a strategic collaboration to revolutionize POC diagnostics by integrating CRISPR technology into POC tests to detect pathogens and genetic markers. Moreover, regional market growth can be attributed to several factors, such as the increasing adoption of NGS, rise in testing of life-threatening diseases, such as cancer, COVID-19, tuberculosis, and STDs, growing demand for personalized medicine, and increasing use of artificial intelligence (AI) & machine learning (ML).
Key Companies & Market Share Insights
Some of the key players operating in the market include F. Hoffmann-La Roche Ltd.; Abbott, Thermo Fisher Scientific Inc.; QIAGEN; and Danaher, among others. These players are adopting different market strategies, such as product launches, collaborations, and partnerships, to increase their industry presence. Market players are launching new molecular diagnostic tests and instruments to chase unmet needs and partnerships to share patented technologies to increase the geographical presence of their existing technologies.
Promega Corporation, Standard BioTools, Co-Diagnostics, Inc., T2 Biosystems, and Huwel Lifesciences are some of the emerging market participants in the molecular diagnostics market. Companies are increasing investment in the research & development of novel products for different end-use applications. Moreover, emerging players are getting financial aid from government and non-government organizations, which is supporting novel product development for the detection of multiple targeted diseases.
Key Molecular Diagnostics Companies:
- BD
- bioMérieux SA
- Bio-Rad Laboratories, Inc.
- Abbott
- Agilent Technologies, Inc.
- Danaher
- Hologic Inc. (Gen Probe)
- Illumina, Inc.
- Grifols, S.A.
- QIAGEN
- F. Hoffmann-La Roche, Ltd.
- Siemens Healthineers AG
- Sysmex Corporation
Recent Developments
-
In January 2024, Illumina collaborated with Janssen to develop a molecular residual disease liquid biopsy test. The company plans to develop a cost-effective whole-genome sequencing multi-cancer test to detect ctDNA
-
In November 2023, Co-Diagnostics, Inc. received a grant of 8.97 million from the Bill & Melinda Gates Foundation to develop a novel tuberculosis test to be run on the Co-Dx PCR platform
-
In July 2023, T2 Biosystems received the Breakthrough Device designation from the U.S. FDA for its C. Auris direct-from-blood molecular diagnostic test
-
In March 2023, F. Hoffmann-La Roche Ltd. and Lilly announced their partnership to improve early Alzheimer’s disease diagnosis
-
In February 2023, Thermo Fisher Scientific Inc. partnered with MyLab to procure RT-PCR kits for various infectious diseases, such as tuberculosis & HIV
-
In January 2023, Agilent Technologies Inc. and Quest Diagnostics to launch ctDx FIRST liquid biopsy NGS test in the U.S.
Molecular Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 13.78 billion
Revenue forecast in 2030
USD 17.97 billion
Growth rate
CAGR of 4.5% from 2024 to 2030
Actual data
2018 - 2023
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Products, technology, application, test location, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Spain; Italy; Belgium; Switzerland; The Netherlands; Poland; Austria; Denmark; Sweden; Turkey; Norway; Japan; China; India; Australia; South Korea; New Zealand; Sri Lanka; Malaysia; Thailand; Vietnam; Singapore; Brazil; Mexico; Colombia; Chile; Peru; Argentina; South Africa; Saudi Arabia; Jordan; UAE; Qatar; Kuwait; Egypt
Key companies profiled
BD; bioMérieux SA; Bio-Rad Laboratories, Inc.; Abbott; Agilent Technologies, Inc.; Danaher; Hologic Inc. (Gen Probe); Illumina, Inc.; Grifols, S.A.; QIAGEN; F. Hoffmann-La Roche, Ltd.; Siemens Healthineers AG; Sysmex Corporation
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Molecular Diagnostics Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the molecular diagnostics market report based on product, technology, application, test location, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Instruments
-
Reagents
-
Others
-
-
Technology Outlook (Revenue, USD Million, 2018 - 2030)
-
Polymerase chain reaction (PCR)
-
By Type
-
Multiplex PCR
-
Other PCR
-
-
By Product
-
Instruments
-
Reagents
-
Others
-
-
-
In Situ Hybridization (ISH)
-
Instruments
-
Reagents
-
Others
-
-
Isothermal Nucleic Acid Amplification Technology (INAAT)
-
Instruments
-
Reagents
-
Others
-
-
Chips and Microarrays
-
Instruments
-
Reagents
-
Others
-
-
Mass Spectrometry
-
Instruments
-
Reagents
-
Others
-
-
Sequencing
-
Instruments
-
Reagents
-
Others
-
-
Transcription Mediated Amplification (TMA)
-
Instruments
-
Reagents
-
Others
-
-
Others
-
Instruments
-
Reagents
-
Others
-
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Oncology
-
Breast Cancer
-
Prostate Cancer
-
Colorectal Cancer
-
Cervical Cancer
-
Kidney Cancer
-
Liver Cancer
-
Blood Cancer
-
Lung Cancer
-
Other Cancer
-
-
Pharmacogenomics
-
Infectious Diseases
-
Methicillin-resistant Staphylococcus Aureus (MRSA)
-
Clostridium Difficile
-
Vancomycin-resistant Enterococci (VRE)
-
Carbapenem-resistant Bacteria
-
Flu
-
Respiratory Syncytial Virus (RSV)
-
Candida
-
Tuberculosis and Drug-resistant TBA
-
Meningitis
-
Gastrointestinal Panel Testing
-
Chlamydia
-
Gonorrhea
-
HIV
-
Hepatitis C
-
Hepatitis B
-
Other Infectious Diseases
-
-
Genetic Testing
-
Newborn Screening
-
Predictive and Presymptomatic Testing
-
Other Genetic Testing
-
-
Neurological Disease
-
Cardiovascular Disease
-
Microbiology
-
Others
-
-
Test Location Outlook (Revenue, USD Million, 2018 - 2030)
-
Point of Care
-
Self-test or OTC
-
Central Laboratories
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Denmark
-
Norway
-
Belgium
-
Switzerland
-
The Netherlands
-
Poland
-
Austria
-
Turkey
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
South Korea
-
Thailand
-
New Zealand
-
Sri Lanka
-
Malaysia
-
Vietnam
-
Singapore
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
Colombia
-
Chile
-
Peru
-
-
Middle East and Africa (MEA)
-
Saudi Arabia
-
UAE
-
South Africa
-
Kuwait
-
Jordan
-
Qatar
-
Egypt
-
-
Frequently Asked Questions About This Report
b. The global molecular diagnostics market size was estimated at USD 15.20 billion in 2023 and is expected to reach USD 13.78 billion in 2024.
b. The global molecular diagnostics market is expected to witness a compounded annual rate of 4.5% from 2024 to 2030 to reach USD 17.97 billion by 2030.
b. The reagents segment accounted for the largest revenue share of 62.2% in 2023 in the molecular diagnostics market. It is anticipated to grow at a significant rate in the coming years, owing to its wide adoption in research and clinical settings.
b. The central laboratories segment dominated the molecular diagnostics market and accounted for the largest revenue share of 77.1% in 2023. This growth is attributable to the high market penetration and test volumes.
b. The Polymerase Chain Reaction (PCR) segment dominated the molecular diagnostics market and accounted for the largest revenue share of 49.6% in 2023. This is attributed to its use in the detection of Covid-19.
Table of Contents
Chapter 1. Methodology and Scope
1.1. Market Segmentation and Scope
1.2. Market Definitions
1.2.1. Product
1.2.2. Technology
1.2.3. Application
1.2.4. Test Location
1.3. Regional Scope
1.4. Estimates and Forecast Timeline
1.5. Research Methodology
1.6. Information Procurement
1.6.1. Purchased Database
1.6.2. GVR’s Internal Database
1.7. Details of primary research
1.8. Market Formulation & Validation
1.9. Model Details
1.9.1. Commodity flow analysis (Model 1)
1.9.1.1. Approach 1: Commodity flow approach
1.9.2. Volume price analysis (Model 2)
1.9.2.1. Approach 2: Volume price analysis
1.10. Research Scope and Assumptions
1.10.1. List of Secondary Sources
1.10.2. List of Primary Sources
1.10.3. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.3. Competitive Insights
Chapter 3. Molecular Diagnostics Market Variables, Trends, & Scope
3.1. Market Lineage Outlook
3.1.1. Parent Market Outlook
3.1.2. Related/Ancillary Market Outlook
3.2. Market Dynamics
3.2.1. Market Drivers Analysis
3.2.1.1. Increase in geriatric population
3.2.1.2. Introduction of technologically advanced products
3.2.1.3. Increase in demand for point-of-care Testing
3.2.1.4. Growing prevalence of target diseases
3.2.1.5. Increasing external funding for R&D
3.2.2. Market Restraints Analysis
3.2.2.1. Presence of ambiguous regulatory framework
3.2.2.2. High prices of molecular diagnostics tests
3.3. Molecular Diagnostics Market Analysis Tools
3.3.1. Porter’s Analysis
3.3.1.1. Bargaining power of the suppliers
3.3.1.2. Bargaining power of the buyers
3.3.1.3. Threats of substitution
3.3.1.4. Threats from new entrants
3.3.1.5. Competitive rivalry
3.3.2. PESTEL Analysis
3.3.2.1. Political landscape
3.3.2.2. Economic and Social landscape
3.3.2.3. Technological landscape
3.3.2.4. Legal landscape
3.4. Market Opportunity Analysis
3.5. CLIA-Waived Tests and Instruments
3.6. Pricing Analysis
Chapter 4. Molecular Diagnostics Market: Product Estimates & Trend Analysis
4.1. Segment Dashboard
4.2. Molecular Diagnostics Market: Product Movement Analysis, USD Million, 2023 & 2030 (Volume Analysis)
4.3. Instruments
4.3.1. Instruments Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold)
4.4. Reagents
4.4.1. Reagents Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Tests Sold)
4.5. Software
4.5.1. Software Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 5. Molecular Diagnostics Market: Technology Estimates & Trend Analysis
5.1. Technology Market Share, 2023 & 2030
5.2. Segment Dashboard
5.3. Global Molecular Diagnostics Market by Technology Outlook
5.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
5.4.1. Polymerase chain reaction (PCR)
5.4.1.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.1.2. PCR, by Type
5.4.1.2.1. Multiplex PCR
5.4.1.2.1.1. Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.1.2.2. Other PCR
5.4.1.2.2.1. Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.1.3. PCR, by Product
5.4.1.3.1. Instruments
5.4.1.3.1.1. Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold)
5.4.1.3.2. Reagents
5.4.1.3.2.1. Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Tests Sold)
5.4.1.3.3. Others
5.4.1.3.3.1. Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.2. In Situ Hybridization (ISH)
5.4.2.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.2.2. Instruments
5.4.2.2.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Instruments Sold)
5.4.2.3. Reagents
5.4.2.3.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Tests Sold)
5.4.2.4. Others
5.4.2.4.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.3. Isothermal Nucleic Acid Amplification Technology (INAAT)
5.4.3.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.3.2. Instruments
5.4.3.2.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Instruments Sold)
5.4.3.3. Reagents
5.4.3.3.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Tests Sold)
5.4.3.4. Others
5.4.3.4.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.4. Chips and Microarrays
5.4.4.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.4.2. Instruments
5.4.4.2.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Instruments Sold)
5.4.4.3. Reagents
5.4.4.3.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Tests Sold)
5.4.4.4. Others
5.4.4.4.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.5. Mass Spectrometry
5.4.5.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.5.2. Instruments
5.4.5.2.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Instruments Sold)
5.4.5.3. Reagents
5.4.5.3.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Tests Sold)
5.4.5.4. Others
5.4.5.4.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.6. Sequencing
5.4.6.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.6.2. Instruments
5.4.6.2.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Instruments Sold)
5.4.6.3. Reagents
5.4.6.3.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Tests Sold)
5.4.6.4. Others
5.4.6.4.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.7. Transcription Mediated Amplification (TMA)
5.4.7.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.7.2. Instruments
5.4.7.2.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Instruments Sold)
5.4.7.3. Reagents
5.4.7.3.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Tests Sold)
5.4.7.4. Others
5.4.7.4.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.8. Others
5.4.8.1. Market estimates and forecasts 2018 to 2030 (USD Million)
5.4.8.2. Instruments
5.4.8.2.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Instruments Sold)
5.4.8.3. Reagents
5.4.8.3.1. Market estimates and forecasts 2018 to 2030 (USD Million) (Volume, Number of Tests Sold)
5.4.8.4. Services
5.4.8.4.1. Market estimates and forecasts 2018 to 2030 (USD Million)
Chapter 6. Molecular Diagnostics Market: Application Estimates & Trend Analysis
6.1. Segment Dashboard
6.2. Molecular Diagnostics Market: Application Movement Analysis, USD Million, 2023 & 2030
6.3. Oncology
6.3.1. Oncology Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.3.2. Breast Cancer
6.3.2.1. Breast Cancer Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.3.3. Prostate Cancer
6.3.3.1. Prostate Cancer Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.3.4. Colorectal Cancer
6.3.4.1. Colorectal Cancer Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.3.5. Cervical Cancer
6.3.5.1. Cervical Cancer Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.3.6. Kidney Cancer
6.3.6.1. Kidney Cancer Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.3.7. Liver Cancer
6.3.7.1. Liver Cancer Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.3.8. Blood Cancer
6.3.8.1. Blood Cancer Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.3.9. Lung Cancer
6.3.9.1. Lung Cancer Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.3.10. Other Cancer
6.3.10.1. Other Cancer Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.4. Pharmacogenomics
6.4.1. Pharmacogenomics Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5. Infectious Diseases
6.5.1. Infectious Diseases Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.2. Methicillin-resistant Staphylococcus Aureus (MRSA)
6.5.2.1. Methicillin-resistant Staphylococcus Aureus (MRSA) Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.3. Clostridium Difficile
6.5.3.1. Clostridium Difficile Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.4. Vancomycin-resistant Enterococci (VRE)
6.5.4.1. Vancomycin-resistant Enterococci (VRE) Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.5. Carbapenem-resistant Bacteria
6.5.5.1. Carbapenem-resistant Bacteria Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.6. Flu
6.5.6.1. Flu Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.7. Respiratory Syncytial Virus (RSV)
6.5.7.1. Respiratory Syncytial Virus (RSV) Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.8. Candida
6.5.8.1. Candida Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.9. Tuberculosis and Drug-resistant TBA
6.5.9.1. Tuberculosis and Drug-resistant TBA Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.10. Meningitis
6.5.10.1. Meningitis Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.11. Gastrointestinal Panel Testing
6.5.11.1. Gastrointestinal Panel Testing Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.12. Chlamydia
6.5.12.1. Chlamydia Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.13. Gonorrhea
6.5.13.1. Gonorrhea Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.14. HIV
6.5.14.1. HIV Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.15. Hepatitis C
6.5.15.1. Hepatitis C Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.16. Hepatitis B
6.5.16.1. Hepatitis B Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.17. Other Infectious Disease
6.5.17.1. Other Infectious Disease Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.6. Genetic Testing
6.6.1. Genetic Testing Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.6.2. Newborn Screening
6.6.2.1. Newborn Screening Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.6.3. Predictive and Presymptomatic Testing
6.6.3.1. Predictive and Presymptomatic Testing Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.6.4. Other Genetic Testing
6.6.4.1. Other Genetic Testing Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.7. Neurological Disease
6.7.1. Neurological Disease Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.8. Cardiovascular Disease
6.8.1. Cardiovascular Disease Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.9. Microbiology
6.9.1. Microbiology Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.10. Others
6.10.1. Others Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 7. Molecular Diagnostics Market: Test Location Estimates & Trend Analysis
7.1. Segment Dashboard
7.2. Molecular Diagnostics Market: Test Location Movement Analysis, USD Million, 2023 & 2030
7.3. Point of Care
7.3.1. Point of Care Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.4. Central Laboratories
7.4.1. Central Laboratories Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.5. Self-Test or OTC
7.5.1. Self-Test or OTC Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 8. Molecular Diagnostics Market: Regional Estimates & Trend Analysis
8.1. Molecular Diagnostics Market Share, By Region, 2023 & 2030, USD Million
8.2. North America
8.2.1. North America Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.2.2. U.S.
8.2.2.1. Key Country Dynamics
8.2.2.2. Regulatory Landscape/Reimbursement Scenario
8.2.2.3. Competitive Insights
8.2.3. U.S. Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.2.4. Canada
8.2.4.1. Key Country Dynamics
8.2.4.2. Regulatory Landscape/Reimbursement Scenario
8.2.4.3. Competitive Insights
8.2.4.4. Canada Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3. Europe
8.3.1. Europe Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.2. UK
8.3.2.1. Key Country Dynamics
8.3.2.2. Regulatory Landscape/Reimbursement Scenario
8.3.2.3. Competitive Insights
8.3.2.4. UK Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.3. Germany
8.3.3.1. Key Country Dynamics
8.3.3.2. Regulatory Landscape/Reimbursement Scenario
8.3.3.3. Competitive Insights
8.3.3.4. Germany Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.4. France
8.3.4.1. Key Country Dynamics
8.3.4.2. Regulatory Landscape/Reimbursement Scenario
8.3.4.3. Competitive Insights
8.3.4.4. France Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.5. Italy
8.3.5.1. Key Country Dynamics
8.3.5.2. Regulatory Landscape/Reimbursement Scenario
8.3.5.3. Competitive Insights
8.3.5.4. Italy Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.6. Spain
8.3.6.1. Key Country Dynamics
8.3.6.2. Regulatory Landscape/Reimbursement Scenario
8.3.6.3. Competitive Insights
8.3.6.4. Spain Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.7. Sweden
8.3.7.1. Key Country Dynamics
8.3.7.2. Regulatory Landscape/Reimbursement Scenario
8.3.7.3. Competitive Insights
8.3.7.4. Sweden Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.8. Denmark
8.3.8.1. Key Country Dynamics
8.3.8.2. Regulatory Landscape/Reimbursement Scenario
8.3.8.3. Competitive Insights
8.3.8.4. Denmark Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.9. Norway
8.3.9.1. Key Country Dynamics
8.3.9.2. Regulatory Landscape/Reimbursement Scenario
8.3.9.3. Competitive Insights
8.3.9.4. Norway Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.10. Belgium
8.3.10.1. Key Country Dynamics
8.3.10.2. Regulatory Landscape/Reimbursement Scenario
8.3.10.3. Competitive Insights
8.3.10.4. Belgium Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.11. Switzerland
8.3.11.1. Key Country Dynamics
8.3.11.2. Regulatory Landscape/Reimbursement Scenario
8.3.11.3. Competitive Insights
8.3.11.4. Switzerland Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.12. The Netherlands
8.3.12.1. Key Country Dynamics
8.3.12.2. Regulatory Landscape/Reimbursement Scenario
8.3.12.3. Competitive Insights
8.3.12.4. The Netherlands Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.13. Poland
8.3.13.1. Key Country Dynamics
8.3.13.2. Regulatory Landscape/Reimbursement Scenario
8.3.13.3. Competitive Insights
8.3.13.4. Poland Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.3.14. Austria
8.3.14.1. Key Country Dynamics
8.3.14.2. Regulatory Landscape/Reimbursement Scenario
8.3.14.3. Competitive Insights
8.3.14.4. Austria Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.3.15. Turkey
8.3.15.1. Key Country Dynamics
8.3.15.2. Regulatory Landscape/Reimbursement Scenario
8.3.15.3. Competitive Insights
8.3.15.4. Turkey Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4. Asia Pacific
8.4.1. Asia Pacific Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4.2. China
8.4.2.1. Key Country Dynamics
8.4.2.2. Regulatory Landscape/Reimbursement Scenario
8.4.2.3. Competitive Insights
8.4.2.4. China Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4.3. Japan
8.4.3.1. Key Country Dynamics
8.4.3.2. Regulatory Landscape/Reimbursement Scenario
8.4.3.3. Competitive Insights
8.4.3.4. Japan Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4.4. India
8.4.4.1. Key Country Dynamics
8.4.4.2. Regulatory Landscape/Reimbursement Scenario
8.4.4.3. Competitive Insights
8.4.4.4. India Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4.5. South Korea
8.4.5.1. Key Country Dynamics
8.4.5.2. Regulatory Landscape/Reimbursement Scenario
8.4.5.3. Competitive Insights
8.4.5.4. South Korea Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4.6. Australia
8.4.6.1. Key Country Dynamics
8.4.6.2. Regulatory Landscape/Reimbursement Scenario
8.4.6.3. Competitive Insights
8.4.6.4. Australia Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4.7. Thailand
8.4.7.1. Key Country Dynamics
8.4.7.2. Regulatory Landscape/Reimbursement Scenario
8.4.7.3. Competitive Insights
8.4.7.4. Thailand Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4.8. New Zealand
8.4.8.1. Key Country Dynamics
8.4.8.2. Regulatory Landscape/Reimbursement Scenario
8.4.8.3. Competitive Insights
8.4.8.4. New Zealand Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4.9. Sri Lanka
8.4.9.1. Key Country Dynamics
8.4.9.2. Regulatory Landscape/Reimbursement Scenario
8.4.9.3. Competitive Insights
8.4.9.4. Sri Lanka Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4.10. Malaysia
8.4.10.1. Key Country Dynamics
8.4.10.2. Regulatory Landscape/Reimbursement Scenario
8.4.10.3. Competitive Insights
8.4.10.4. Malaysia Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4.11. Vietnam
8.4.11.1. Key Country Dynamics
8.4.11.2. Regulatory Landscape/Reimbursement Scenario
8.4.11.3. Competitive Insights
8.4.11.4. Vietnam Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.4.12. Singapore
8.4.12.1. Key Country Dynamics
8.4.12.2. Regulatory Landscape/Reimbursement Scenario
8.4.12.3. Competitive Insights
8.4.12.4. Singapore Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.5. Latin America
8.5.1. Latin America Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.5.2. Brazil
8.5.2.1. Key Country Dynamics
8.5.2.2. Regulatory Landscape/Reimbursement Scenario
8.5.2.3. Competitive Insights
8.5.2.4. Brazil Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.5.3. Mexico
8.5.3.1. Key Country Dynamics
8.5.3.2. Regulatory Landscape/Reimbursement Scenario
8.5.3.3. Competitive Insights
8.5.3.4. Mexico Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.5.4. Argentina
8.5.4.1. Key Country Dynamics
8.5.4.2. Regulatory Landscape/Reimbursement Scenario
8.5.4.3. Competitive Insights
8.5.4.4. Argentina Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.5.5. Colombia
8.5.5.1. Key Country Dynamics
8.5.5.2. Regulatory Landscape/Reimbursement Scenario
8.5.5.3. Competitive Insights
8.5.5.4. Colombia Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.5.6. Chile
8.5.6.1. Key Country Dynamics
8.5.6.2. Regulatory Landscape/Reimbursement Scenario
8.5.6.3. Competitive Insights
8.5.6.4. Chile Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.5.7. Peru
8.5.7.1. Key Country Dynamics
8.5.7.2. Regulatory Landscape/Reimbursement Scenario
8.5.7.3. Competitive Insights
8.5.7.4. Peru Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.6. Middle East and Africa
8.6.1. Middle East and Africa Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.6.2. Saudi Arabia
8.6.2.1. Key Country Dynamics
8.6.2.2. Regulatory Landscape/Reimbursement Scenario
8.6.2.3. Competitive Insights
8.6.2.4. Saudi Arabia Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.6.3. UAE
8.6.3.1. Key Country Dynamics
8.6.3.2. Regulatory Landscape/Reimbursement Scenario
8.6.3.3. Competitive Insights
8.6.3.4. UAE Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.6.4. South Africa
8.6.4.1. Key Country Dynamics
8.6.4.2. Regulatory Landscape/Reimbursement Scenario
8.6.4.3. Competitive Insights
8.6.4.4. South Africa Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.6.5. Kuwait
8.6.5.1. Key Country Dynamics
8.6.5.2. Regulatory Landscape/Reimbursement Scenario
8.6.5.3. Competitive Insights
8.6.5.4. Kuwait Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.6.6. Jordan
8.6.6.1. Key Country Dynamics
8.6.6.2. Regulatory Landscape/Reimbursement Scenario
8.6.6.3. Competitive Insights
8.6.6.4. Jordan Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.6.7. Qatar
8.6.7.1. Key Country Dynamics
8.6.7.2. Regulatory Landscape/Reimbursement Scenario
8.6.7.3. Competitive Insights
8.6.7.4. Qatar Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
8.6.8. Egypt
8.6.8.1. Key Country Dynamics
8.6.8.2. Regulatory Landscape/Reimbursement Scenario
8.6.8.3. Competitive Insights
8.6.8.4. Egypt Molecular Diagnostics Market Estimates and Forecasts, 2018 - 2030 (USD Million) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
Chapter 9. Competitive Landscape
9.1. Recent Developments & Impact Analysis by Key Market Participants
9.2. Company Categorization
9.3. Global Company Market Share Analysis for Molecular Diagnostics and PCR, 2023
9.4. North America Company Market Share Analysis for Molecular Diagnostics and PCR, 2023
9.5. Europe Company Market Share Analysis for Molecular Diagnostics and PCR, 2023
9.6. Asia Pacific Company Market Share Analysis for Molecular Diagnostics and PCR, 2023
9.7. Latin America Company Market Share Analysis for Molecular Diagnostics and PCR, 2023
9.8. MEA Company Market Share Analysis for Molecular Diagnostics and PCR, 2023
9.9. Company Heat Map Analysis
9.10. Strategy Mapping
9.10.1. Expansion
9.10.2. Mergers & Acquisition
9.10.3. Partnerships & Collaborations
9.10.4. New Product Launches
9.10.5. Research And Development
9.11. Company Profiles
9.11.1. QIAGEN
9.11.1.1. Participant’s Overview
9.11.1.2. Financial Performance
9.11.1.3. Product Benchmarking
9.11.1.4. Recent Developments
9.11.2. BD
9.11.2.1. Participant’s Overview
9.11.2.2. Financial Performance
9.11.2.3. Product Benchmarking
9.11.2.4. Recent Developments
9.11.3. bioMérieux SA
9.11.3.1. Participant’s Overview
9.11.3.2. Financial Performance
9.11.3.3. Product Benchmarking
9.11.3.4. Recent Developments
9.11.4. F. Hoffmann-La Roche, Ltd.
9.11.4.1. Participant’s Overview
9.11.4.2. Financial Performance
9.11.4.3. Product Benchmarking
9.11.4.4. Recent Developments
9.11.5. Hologic, Inc. (Gen Probe)
9.11.5.1. Participant’s Overview
9.11.5.2. Financial Performance
9.11.5.3. Product Benchmarking
9.11.5.4. Recent Developments
9.11.6. Abbott
9.11.6.1. Participant’s Overview
9.11.6.2. Financial Performance
9.11.6.3. Product Benchmarking
9.11.6.4. Recent Developments
9.11.7. Agilent Technologies, Inc.
9.11.7.1. Participant’s Overview
9.11.7.2. Financial Performance
9.11.7.3. Product Benchmarking
9.11.7.4. Recent Developments
9.11.8. Siemens Healthineers AG
9.11.8.1. Participant’s Overview
9.11.8.2. Financial Performance
9.11.8.3. Product Benchmarking
9.11.8.4. Recent Developments
9.11.9. Bio-Rad Laboratories, Inc.
9.11.9.1. Participant’s Overview
9.11.9.2. Financial Performance
9.11.9.3. Product Benchmarking
9.11.9.4. Recent Developments
9.11.10. Danaher
9.11.10.1. Participant’s Overview
9.11.10.2. Financial Performance
9.11.10.3. Product Benchmarking
9.11.10.4. Recent Developments
9.11.11. Illumina, Inc.
9.11.11.1. Participant’s Overview
9.11.11.2. Financial Performance
9.11.11.3. Product Benchmarking
9.11.11.4. Recent Developments
List of Tables
Table 1 Country share estimation
Table 2 North America molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 3 North America molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 4 North America molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 5 North America PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 6 North America PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 7 North America ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 8 North America INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 9 North America chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 10 North America mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 11 North America sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 12 North America TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 13 North America others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 14 North America molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 15 North America molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 16 North America molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 17 North America molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 18 U.S. molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 19 U.S. molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 20 U.S. molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 21 U.S. PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 22 U.S. PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 23 U.S. ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 24 U.S. INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 25 U.S. chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 26 U.S. mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 27 U.S. sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 28 U.S. TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 29 U.S. others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 30 U.S. molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 31 U.S. molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 32 U.S. molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 33 U.S. molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 34 Canada molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 35 Canada molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 36 Canada molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 37 Canada PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 38 Canada PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 39 Canada ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 40 Canada INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 41 Canada chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 42 Canada mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 43 Canada sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 44 Canada TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 45 Canada others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 46 Canada molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 47 Canada molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 48 Canada molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 49 Canada molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 50 Europe molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 51 Europe molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 52 Europe molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 53 Europe PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 54 Europe PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 55 Europe ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 56 Europe INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 57 Europe chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 58 Europe mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 59 Europe sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 60 Europe TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 61 Europe others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 62 Europe molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 63 Europe molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 64 Europe molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 65 Europe molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 66 UK molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 67 UK molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 68 UK molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 69 UK PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 70 UK PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 71 UK ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 72 UK INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 73 UK chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 74 UK mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 75 UK sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 76 UK TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 77 UK others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 78 UK molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 79 UK molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 80 UK molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 81 UK molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 82 Germany molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 83 Germany molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 84 Germany molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 85 Germany PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 86 Germany PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 87 Germany ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 88 Germany INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 89 Germany chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 90 Germany mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 91 Germany sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 92 Germany TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 93 Germany others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 94 Germany molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 95 Germany molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 96 Germany molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 97 Germany molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 98 France molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 99 France molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 100 France molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 101 France PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 102 France PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 103 France ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 104 France INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 105 France chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 106 France mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 107 France sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 108 France TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 109 France others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 110 France molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 111 France molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 112 France molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 113 France molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 114 Spain molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 115 Spain molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 116 Spain molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 117 Spain PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 118 Spain PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 119 Spain ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 120 Spain INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 121 Spain chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 122 Spain mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 123 Spain sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 124 Spain TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 125 Spain others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 126 Spain molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 127 Spain molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 128 Spain molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 129 Spain molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 130 Italy molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 131 Italy molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 132 Italy molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 133 Italy PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 134 Italy PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 135 Italy ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 136 Italy INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 137 Italy chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 138 Italy mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 139 Italy sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 140 Italy TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 141 Italy others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 142 Italy molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 143 Italy molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 144 Italy molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 145 Italy molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 146 Belgium molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 147 Belgium molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 148 Belgium molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 149 Belgium PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 150 Belgium PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 151 Belgium ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 152 Belgium INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 153 Belgium chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 154 Belgium mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 155 Belgium sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 156 Belgium TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 157 Belgium others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 158 Belgium molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 159 Belgium molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 160 Belgium molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 161 Belgium molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 162 Switzerland molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 163 Switzerland molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 164 Switzerland molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 165 Switzerland PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 166 Switzerland PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 167 Switzerland ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 168 Switzerland INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 169 Switzerland chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 170 Switzerland mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 171 Switzerland sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 172 Switzerland TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 173 Switzerland others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 174 Switzerland molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 175 Switzerland molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 176 Switzerland molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 177 Switzerland molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 178 Netherlands molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 179 Netherlands molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 180 Netherlands molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 181 Netherlands PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 182 Netherlands PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 183 Netherlands ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 184 Netherlands INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 185 Netherlands chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 186 Netherlands mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 187 Netherlands sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 188 Netherlands TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 189 Netherlands others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 190 Netherlands molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 191 Netherlands molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 192 Netherlands molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 193 Netherlands molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 194 Poland molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 195 Poland molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 196 Poland molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 197 Poland PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 198 Poland PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 199 Poland ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 200 Poland INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 201 Poland chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 202 Poland mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 203 Poland sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 204 Poland TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 205 Poland others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 206 Poland molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 207 Poland molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 208 Poland molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 209 Poland molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 210 Austria molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 211 Austria molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 212 Austria molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 213 Austria PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 214 Austria PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 215 Austria ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 216 Austria INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 217 Austria chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 218 Austria mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 219 Austria sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 220 Austria TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 221 Austria others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 222 Austria molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 223 Austria molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 224 Austria molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 225 Austria molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 226 Denmark molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 227 Denmark molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 228 Denmark molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 229 Denmark PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 230 Denmark PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 231 Denmark ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 232 Denmark INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 233 Denmark chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 234 Denmark mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 235 Denmark sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 236 Denmark TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 237 Denmark others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 238 Denmark molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 239 Denmark molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 240 Denmark molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 241 Denmark molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 242 Sweden molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 243 Sweden molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 244 Sweden molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 245 Sweden PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 246 Sweden PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 247 Sweden ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 248 Sweden INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 249 Sweden chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 250 Sweden mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 251 Sweden sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 252 Sweden TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 253 Sweden others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 254 Sweden molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 255 Sweden molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 256 Sweden molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 257 Sweden molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 258 Turkey molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 259 Turkey molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 260 Turkey molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 261 Turkey PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 262 Turkey PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 263 Turkey ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 264 Turkey INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 265 Turkey chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 266 Turkey mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 267 Turkey sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 268 Turkey TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 269 Turkey others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 270 Turkey molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 271 Turkey molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 272 Turkey molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 273 Turkey molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 274 Norway molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 275 Norway molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 276 Norway molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 277 Norway PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 278 Norway PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 279 Norway ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 280 Norway INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 281 Norway chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 282 Norway mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 283 Norway sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 284 Norway TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 285 Norway others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 286 Norway molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 287 Norway molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 288 Norway molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 289 Norway molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 290 Asia Pacific molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 291 Asia Pacific molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 292 Asia Pacific molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 293 Asia Pacific PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 294 Asia Pacific PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 295 Asia Pacific ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 296 Asia Pacific INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 297 Asia Pacific chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 298 Asia Pacific mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 299 Asia Pacific sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 300 Asia Pacific TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 301 Asia Pacific others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 302 Asia Pacific molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 303 Asia Pacific molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 304 Asia Pacific molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 305 Asia Pacific molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 306 Japan molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 307 Japan molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 308 Japan molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 309 Japan PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 310 Japan PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 311 Japan ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 312 Japan INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 313 Japan chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 314 Japan mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 315 Japan sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 316 Japan TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 317 Japan others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 318 Japan molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 319 Japan molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 320 Japan molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 321 Japan molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 322 China molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 323 China molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 324 China molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 325 China PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 326 China PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 327 China ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 328 China INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 329 China chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 330 China mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 331 China sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 332 China TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 333 China others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 334 China molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 335 China molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 336 China molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 337 China molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 338 India molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 339 India molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 340 India molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 341 India PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 342 India PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 343 India ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 344 India INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 345 India chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 346 India mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 347 India sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 348 India TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 349 India others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 350 India molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 351 India molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 352 India molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 353 India molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 354 Australia molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 355 Australia molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 356 Australia molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 357 Australia PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 358 Australia PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 359 Australia ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 360 Australia INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 361 Australia chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 362 Australia mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 363 Australia sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 364 Australia TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 365 Australia others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 366 Australia molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 367 Australia molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 368 Australia molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 369 Australia molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 370 New Zealand molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 371 New Zealand molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 372 New Zealand molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 373 New Zealand PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 374 New Zealand PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 375 New Zealand ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 376 New Zealand INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 377 New Zealand chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 378 New Zealand mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 379 New Zealand sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 380 New Zealand TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 381 New Zealand others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 382 New Zealand molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 383 New Zealand molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 384 New Zealand molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 385 New Zealand molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 386 Sri Lanka molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 387 Sri Lanka molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 388 Sri Lanka molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 389 Sri Lanka PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 390 Sri Lanka PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 391 Sri Lanka ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 392 Sri Lanka INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 393 Sri Lanka chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 394 Sri Lanka mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 395 Sri Lanka sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 396 Sri Lanka TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 397 Sri Lanka others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 398 Sri Lanka molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 399 Sri Lanka molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 400 Sri Lanka molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 401 Sri Lanka molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 402 Malaysia molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 403 Malaysia molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 404 Malaysia molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 405 Malaysia PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 406 Malaysia PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 407 Malaysia ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 408 Malaysia INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 409 Malaysia chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 410 Malaysia mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 411 Malaysia sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 412 Malaysia TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 413 Malaysia others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 414 Malaysia molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 415 Malaysia molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 416 Malaysia molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 417 Malaysia molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 418 Thailand molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 419 Thailand molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 420 Thailand molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 421 Thailand PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 422 Thailand PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 423 Thailand ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 424 Thailand INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 425 Thailand chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 426 Thailand mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 427 Thailand sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 428 Thailand TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 429 Thailand others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 430 Thailand molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 431 Thailand molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 432 Thailand molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 433 Thailand molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 434 Vietnam molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 435 Vietnam molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 436 Vietnam molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 437 Vietnam PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 438 Vietnam PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 439 Vietnam ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 440 Vietnam INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 441 Vietnam chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 442 Vietnam mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 443 Vietnam sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 444 Vietnam TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 445 Vietnam others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 446 Vietnam molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 447 Vietnam molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 448 Vietnam molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 449 Vietnam molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 450 Singapore molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 451 Singapore molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 452 Singapore molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 453 Singapore PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 454 Singapore PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 455 Singapore ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 456 Singapore INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 457 Singapore chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 458 Singapore mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 459 Singapore sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 460 Singapore TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 461 Singapore others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 462 Singapore molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 463 Singapore molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 464 Singapore molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 465 Singapore molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 466 South Korea molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 467 South Korea molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 468 South Korea molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 469 South Korea PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 470 South Korea PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 471 South Korea ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 472 South Korea INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 473 South Korea chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 474 South Korea mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 475 South Korea sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 476 South Korea TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 477 South Korea others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 478 South Korea molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 479 South Korea molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 480 South Korea molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 481 South Korea molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 482 Latin America molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 483 Latin America molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 484 Latin America molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 485 Latin America PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 486 Latin America PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 487 Latin America ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 488 Latin America INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 489 Latin America chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 490 Latin America mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 491 Latin America sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 492 Latin America TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 493 Latin America others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 494 Latin America molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 495 Latin America molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 496 Latin America molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 497 Latin America molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 498 Brazil molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 499 Brazil molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 500 Brazil molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 501 Brazil PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 502 Brazil PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 503 Brazil ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 504 Brazil INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 505 Brazil chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 506 Brazil mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 507 Brazil sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 508 Brazil TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 509 Brazil others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 510 Brazil molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 511 Brazil molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 512 Brazil molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 513 Brazil molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 514 Mexico molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 515 Mexico molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 516 Mexico molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 517 Mexico PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 518 Mexico PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 519 Mexico ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 520 Mexico INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 521 Mexico chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 522 Mexico mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 523 Mexico sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 524 Mexico TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 525 Mexico others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 526 Mexico molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 527 Mexico molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 528 Mexico molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 529 Mexico molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 530 Colombia molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 531 Colombia molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 532 Colombia molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 533 Colombia PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 534 Colombia PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 535 Colombia ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 536 Colombia INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 537 Colombia chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 538 Colombia mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 539 Colombia sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 540 Colombia TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 541 Colombia others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 542 Colombia molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 543 Colombia molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 544 Colombia molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 545 Colombia molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 546 Chile molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 547 Chile molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 548 Chile molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 549 Chile PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 550 Chile PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 551 Chile ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 552 Chile INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 553 Chile chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 554 Chile mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 555 Chile sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 556 Chile TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 557 Chile others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 558 Chile molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 559 Chile molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 560 Chile molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 561 Chile molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 562 Peru molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 563 Peru molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 564 Peru molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 565 Peru PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 566 Peru PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 567 Peru ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 568 Peru INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 569 Peru chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 570 Peru mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 571 Peru sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 572 Peru TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 573 Peru others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 574 Peru molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 575 Peru molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 576 Peru molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 577 Peru molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 578 Argentina molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 579 Argentina molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 580 Argentina molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 581 Argentina PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 582 Argentina PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 583 Argentina ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 584 Argentina INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 585 Argentina chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 586 Argentina mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 587 Argentina sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 588 Argentina TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 589 Argentina others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 590 Argentina molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 591 Argentina molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 592 Argentina molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 593 Argentina molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 594 MEA molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 595 MEA molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 596 MEA molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 597 MEA PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 598 MEA PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 599 MEA ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 600 MEA INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 601 MEA chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 602 MEA mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 603 MEA sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 604 MEA TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 605 MEA others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 606 MEA molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 607 MEA molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 608 MEA molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 609 MEA molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 610 South Africa molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 611 South Africa molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 612 South Africa molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 613 South Africa PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 614 South Africa PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 615 South Africa ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 616 South Africa INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 617 South Africa chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 618 South Africa mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 619 South Africa sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 620 South Africa TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 621 South Africa others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 622 South Africa molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 623 South Africa molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 624 South Africa molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 625 South Africa molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 626 Saudi Arabia molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 627 Saudi Arabia molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 628 Saudi Arabia molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 629 Saudi Arabia PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 630 Saudi Arabia PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 631 Saudi Arabia ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 632 Saudi Arabia INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 633 Saudi Arabia chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 634 Saudi Arabia mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 635 Saudi Arabia sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 636 Saudi Arabia TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 637 Saudi Arabia others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 638 Saudi Arabia molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 639 Saudi Arabia molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 640 Saudi Arabia molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 641 Saudi Arabia molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 642 Jordan molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 643 Jordan molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 644 Jordan molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 645 Jordan PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 646 Jordan PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 647 Jordan ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 648 Jordan INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 649 Jordan chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 650 Jordan mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 651 Jordan sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 652 Jordan TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 653 Jordan others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 654 Jordan molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 655 Jordan molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 656 Jordan molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 657 Jordan molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 658 UAE molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 659 UAE molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 660 UAE molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 661 UAE PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 662 UAE PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 663 UAE ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 664 UAE INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 665 UAE chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 666 UAE mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 667 UAE sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 668 UAE TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 669 UAE others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 670 UAE molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 671 UAE molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 672 UAE molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 673 UAE molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 674 Qatar molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 675 Qatar molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 676 Qatar molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 677 Qatar PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 678 Qatar PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 679 Qatar ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 680 Qatar INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 681 Qatar chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 682 Qatar mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 683 Qatar sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 684 Qatar TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 685 Qatar others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 686 Qatar molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 687 Qatar molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 688 Qatar molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 689 Qatar molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 690 Kuwait molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 691 Kuwait molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 692 Kuwait molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 693 Kuwait PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 694 Kuwait PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 695 Kuwait ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 696 Kuwait INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 697 Kuwait chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 698 Kuwait mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 699 Kuwait sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 700 Kuwait TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 701 Kuwait others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 702 Kuwait molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 703 Kuwait molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 704 Kuwait molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 705 Kuwait molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
Table 706 Egypt molecular diagnostics market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 707 Egypt molecular diagnostics market estimates and forecasts, by test location, 2018 - 2030 (USD Million)
Table 708 Egypt molecular diagnostics market estimates and forecasts, by technology, 2018 - 2030 (USD Million)
Table 709 Egypt PCR market estimates and forecasts, by type, 2018 - 2030 (USD Million)
Table 710 Egypt PCR market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 711 Egypt ISH market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 712 Egypt INAAT market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 713 Egypt chips and microarrays market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 714 Egypt mass spectrometry market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 715 Egypt sequencing market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 716 Egypt TMA market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 717 Egypt others technology market estimates and forecasts, by product, 2018 - 2030 (USD Million)
Table 718 Egypt molecular diagnostics market estimates and forecasts, by application, 2018 - 2030 (USD Million)
Table 719 Egypt molecular diagnostics market estimates and forecasts, by oncology, 2018 - 2030 (USD Million)
Table 720 Egypt molecular diagnostics market estimates and forecasts, by infectious diseases, 2018 - 2030 (USD Million)
Table 721 Egypt molecular diagnostics market estimates and forecasts, by genetic testing, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Molecular diagnostics market segmentation
Fig. 2 Market research process
Fig. 3 Data triangulation techniques
Fig. 4 Primary research pattern
Fig. 5 Market research approaches
Fig. 6 Value-chain-based sizing & forecasting
Fig. 7 QFD modeling for market share assessment
Fig. 8 Market formulation & validation
Fig. 9 Molecular diagnostics market snapshot
Fig. 10 Parent market outlook
Fig. 11 Penetration & growth prospect mapping
Fig. 12 Market driver relevance analysis (Current & future impact)
Fig. 13 Global population by age group, 2017, 2021, & 2030
Fig. 14 General trend shifts from laboratory to point-of-care
Fig. 15 Market restrain relevance analysis (Current & future impact)
Fig. 16 Porter’s five forces analysis
Fig. 17 SWOT analysis, by factor (Political & legal, economic and technological)
Fig. 18 Strategy mapping
Fig. 19 Competition categorization
Fig. 20 Company market share analysis - Molecular diagnostics market, 2023
Fig. 21 Company market share analysis - Molecular diagnostics, oncology, 2023
Fig. 22 Company market share analysis - Molecular diagnostics, pharmacogenomics, 2023
Fig. 23 Company market share analysis - Molecular diagnostics, infectious diseases, 2023
Fig. 24 Company market share analysis - Molecular diagnostics, genetic testing, 2023
Fig. 25 Company market share analysis - Molecular diagnostics, others (neurological disease, cardiovascular diseases, microbiology, others), 2023
Fig. 26 Company market share analysis - Molecular diagnostics, polymerase chain reaction, 2023
Fig. 27 Company market share analysis - Molecular diagnostics, in situ hybridization, 2023
Fig. 28 Company market share analysis - Molecular diagnostics, INAAT, 2023
Fig. 29 Company market share analysis - Molecular diagnostics, chips & microarrays, 2023
Fig. 30 Company market share analysis - Molecular diagnostics, mass spectrometry, 2023
Fig. 31 Company market share analysis - Molecular diagnostics, sequencing, 2023
Fig. 32 Company market share analysis - Molecular diagnostics, transcription mediated amplification, 2023
Fig. 33 Company market share analysis - Molecular diagnostics, oncology, 2023
Fig. 34 Company market position analysis
Fig. 35 Company market position analysis
Fig. 36 Competitive dashboard analysis
Fig. 37 Regional network map
Fig. 38 Competitive Dashboard Analysis
Fig. 39 Molecular diagnostics market: Products outlook and key takeaways
Fig. 40 Molecular diagnostics market: Products movement analysis
Fig. 41 Instruments market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 42 Reagents market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 43 Others market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 44 Molecular diagnostics market: Test location outlook and key takeaways
Fig. 45 Molecular diagnostics market: Test location movement analysis
Fig. 46 PoC market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 47 Self-test or over the counter market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 48 Central laboratories market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 49 Molecular diagnostics market: Technology outlook and key takeaways
Fig. 50 Molecular diagnostics market: Technology movement analysis
Fig. 51 Polymerase chain reaction (PCR) market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 52 Nucleic acid extraction and purification market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 53 Others market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 54 Multiplex PCR market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 55 Other PCR market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 56 Instruments PCR market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 57 Reagents PCR market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 58 Others PCR market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 59 In situ hybridization (ISH) market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 60 Instruments ISH market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 61 Reagents ISH market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 62 Others ISH market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 63 INAAT market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 64 Instruments INAAT market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 65 Reagents INAAT market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 66 Others INAAT market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 67 Chips and microarrays market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 68 Instruments chips and microarrays market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 69 Reagents chips and microarrays market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 70 Others chips and microarrays market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 71 Mass spectrometry market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 72 Instruments mass spectrometry market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 73 Reagents mass spectrometry market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 74 Others mass spectrometry market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 75 Sequencing market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 76 Instruments sequencing market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 77 Reagents sequencing market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 78 Others sequencing market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 79 Transcription mediated amplification (TMA) market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 80 Instruments TMA market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 81 Reagents TMA market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 82 Others TMA market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 83 Others market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 84 Instruments others market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 85 Reagents others market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 86 Others market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 87 Molecular diagnostics market: Application outlook and key takeaways
Fig. 88 Molecular diagnostics market: Application movement analysis
Fig. 89 Oncology market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 90 Breast cancer market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 91 Prostate cancer market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 92 Colorectal cancer market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 93 Cervical cancer market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 94 Kidney cancer market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 95 Liver cancer market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 96 Blood cancer market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 97 Lung cancer market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 98 Other cancer market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 99 Pharmacogenomics market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 100 Infectious diseases market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 101 MRSA market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 102 lostridium difficile market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 103 VRE market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 104 Carbapenem-resistant bacteria market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 105 Flu market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 106 RSV market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 107 Candida market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 108 TB and drug-resistant TB market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 109 Meningitis cancer market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 110 Gastrointestinal panel testing market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 111 Chlamydia market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 112 Gonorrhea market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 113 HIV market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 114 Hepatitis C market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 115 Hepatitis B market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 116 Other infectious disease market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 117 Genetic testing market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 118 Newborn screening market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 119 Predictive and presymptomatic testing market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 120 Other genetic testing market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 121 Neurological diseases market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 122 Cardiovascular diseases market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 123 Microbiology market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 124 Others market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 125 Molecular diagnostics market: Regional outlook and key takeaways
Fig. 126 Molecular diagnostics market: Regional movement analysis
Fig. 127 North America
Fig. 128 North America, SWOT
Fig. 129 North America market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 130 U.S. key country dynamics
Fig. 131 U.S. market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 132 Canada key country dynamics
Fig. 133 Canada market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 134 Europe
Fig. 135 Europe, SWOT
Fig. 136 Europe market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 137 UK key country dynamics
Fig. 138 UK market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 139 Germany key country dynamics
Fig. 140 Germany market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 141 France key country dynamics
Fig. 142 France market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 143 Spain key country dynamics
Fig. 144 Spain market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 145 Italy key country dynamics
Fig. 146 Italy market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 147 Belgium key country dynamics
Fig. 148 Belgium market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 149 Switzerland key country dynamics
Fig. 150 Switzerland market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 151 Netherlands key country dynamics
Fig. 152 Netherlands market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 153 Poland key country dynamics
Fig. 154 Poland market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 155 Austria key country dynamics
Fig. 156 Austria market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 157 Denmark key country dynamics
Fig. 158 Denmark market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 159 Sweden key country dynamics
Fig. 160 Sweden market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 161 Turkey key country dynamics
Fig. 162 Turkey market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 163 Norway key country dynamics
Fig. 164 Norway market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 165 Asia Pacific
Fig. 166 Asia Pacific, SWOT
Fig. 167 Asia Pacific market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 168 Japan key country dynamics
Fig. 169 Japan market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 170 China key country dynamics
Fig. 171 China. market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 172 India key country dynamics
Fig. 173 India. market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 174 Australia key country dynamics
Fig. 175 Australia market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 176 New Zealand key country dynamics
Fig. 177 New Zealand market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 178 Sri Lanka key country dynamics
Fig. 179 Sri Lanka market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 180 Malaysia key country dynamics
Fig. 181 Malaysia market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 182 Thailand key country dynamics
Fig. 183 Thailand market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 184 Vietnam key country dynamics
Fig. 185 Vietnam market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 186 Singapore key country dynamics
Fig. 187 Singapore market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 188 South Korea key country dynamics
Fig. 189 South Korea market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 190 Latin America
Fig. 191 Latin America, SWOT
Fig. 192 Latin America market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 193 Brazil key country dynamics
Fig. 194 Brazil market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 195 Mexico key country dynamics
Fig. 196 Mexico market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 197 Colombia key country dynamics
Fig. 198 Colombia market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 199 Chile key country dynamics
Fig. 200 Chile market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 201 Peru key country dynamics
Fig. 202 Peru market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 203 Argentina key country dynamics
Fig. 204 Argentina market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 205 Middle East & Africa
Fig. 206 Middle East & Africa, SWOT
Fig. 207 Middle East & Africa market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 208 South Africa key country dynamics
Fig. 209 South Africa market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 210 Saudi Arabia key country dynamics
Fig. 211 Saudi Arabia market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 212 Jordan key country dynamics
Fig. 213 Jordan market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 214 United Arab Emirates key country dynamics
Fig. 215 United Arab Emirates market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 216 Qatar key country dynamics
Fig. 217 Qatar market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 218 Kuwait key country dynamics
Fig. 219 Kuwait market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 220 Egypt key country dynamics
Fig. 221 Egypt market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 222 Strategy Framework
Fig. 223 Participant categorizationWhat questions do you have? Get quick response from our industry experts. Request a Free ConsultationMarket Segmentation
- Molecular Diagnostics Product Outlook (Revenue, USD Million, 2018 - 2030) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
- Instruments
- Reagents
- Others
- Molecular Diagnostics Technology Outlook (Revenue, USD Million, 2018 - 2030)
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Molecular Diagnostics Application Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Molecular Diagnostics Test Location Outlook (Revenue, USD Million, 2018 - 2030)
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Molecular Diagnostics Regional Outlook (Revenue, USD Million, 2018 - 2030) (Volume, Number of Instruments Sold) (Volume, Number of Tests Sold)
- North America
- North America Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- North America Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- North America Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- North America Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- U.S.
- U.S. Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- U.S. Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- U.S. Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- U.S. Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- U.S. Molecular Diagnostics Market, By Product
- Canada
- Canada Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Canada Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Canada Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Canada Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Canada Molecular Diagnostics Market, By Product
- North America Molecular Diagnostics Market, By Product
- Europe
- Europe Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Europe Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Europe Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Europe Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- UK
- UK Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- UK Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- UK Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- UK Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- UK Molecular Diagnostics Market, By Product
- Germany
- Germany Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Germany Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Germany Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Germany Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Germany Molecular Diagnostics Market, By Product
- France
- France Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- France Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- France Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- France Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- France Molecular Diagnostics Market, By Product
- Spain
- Spain Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Spain Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Spain Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Spain Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Spain Molecular Diagnostics Market, By Product
- Italy
- Italy Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Italy Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Italy Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Italy Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Italy Molecular Diagnostics Market, By Product
- Belgium
- Belgium Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Belgium Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Belgium Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Belgium Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Belgium Molecular Diagnostics Market, By Product
- Switzerland
- Switzerland Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Switzerland Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Switzerland Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Switzerland Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Switzerland Molecular Diagnostics Market, By Product
- The Netherlands
- The Netherlands Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- The Netherlands Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- The Netherlands Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- The Netherlands Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- The Netherlands Molecular Diagnostics Market, By Product
- Poland
- Poland Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Poland Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Poland Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Poland Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Poland Molecular Diagnostics Market, By Product
- Austria
- Austria Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Austria Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Austria Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Austria Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Austria Molecular Diagnostics Market, By Product
- Denmark
- Denmark Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Denmark Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Denmark Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Denmark Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Denmark Molecular Diagnostics Market, By Product
- Sweden
- Sweden Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Sweden Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Sweden Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Sweden Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Sweden Molecular Diagnostics Market, By Product
- Turkey
- Turkey Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Turkey Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Turkey Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Turkey Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Turkey Molecular Diagnostics Market, By Product
- Norway
- Norway Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Norway Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Norway Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Norway Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Norway Molecular Diagnostics Market, By Product
- Europe Molecular Diagnostics Market, By Product
- Asia Pacific
- Asia Pacific Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Asia Pacific Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Asia Pacific Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Asia Pacific Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Japan
- Japan Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Japan Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Japan Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Japan Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Japan Molecular Diagnostics Market, By Product
- China
- China Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- China Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- China Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- China Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- China Molecular Diagnostics Market, By Product
- India
- India Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- India Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- India Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- India Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- India Molecular Diagnostics Market, By Product
- Australia
- Australia Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Australia Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Australia Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Australia Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Australia Molecular Diagnostics Market, By Product
- South Korea
- South Korea Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- South Korea Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- South Korea Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- South Korea Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- South Korea Molecular Diagnostics Market, By Product
- New Zealand
- New Zealand Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- New Zealand Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- New Zealand Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- New Zealand Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- New Zealand Molecular Diagnostics Market, By Product
- Sri Lanka
- Sri Lanka Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Sri Lanka Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Sri Lanka Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Sri Lanka Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Sri Lanka Molecular Diagnostics Market, By Product
- Malaysia
- Malaysia Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Malaysia Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Malaysia Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Malaysia Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Malaysia Molecular Diagnostics Market, By Product
- Thailand
- Thailand Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Thailand Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Thailand Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Thailand Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Thailand Molecular Diagnostics Market, By Product
- Vietnam
- Vietnam Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Vietnam Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Vietnam Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Vietnam Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Vietnam Molecular Diagnostics Market, By Product
- Singapore
- Singapore Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Singapore Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Singapore Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Singapore Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Singapore Molecular Diagnostics Market, By Product
- Asia Pacific Molecular Diagnostics Market, By Product
- Latin America
- Latin America Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Latin America Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Latin America Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Latin America Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Brazil
- Brazil Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Brazil Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Brazil Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Brazil Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Brazil Molecular Diagnostics Market, By Product
- Mexico
- Mexico Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Mexico Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Mexico Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Mexico Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Mexico Molecular Diagnostics Market, By Product
- Colombia
- Colombia Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Colombia Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Colombia Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Colombia Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Colombia Molecular Diagnostics Market, By Product
- Chile
- Chile Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Chile Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Chile Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Chile Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Chile Molecular Diagnostics Market, By Product
- Peru
- Peru Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Peru Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Peru Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Peru Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Peru Molecular Diagnostics Market, By Product
- Argentina
- Argentina Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Argentina Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Argentina Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Argentina Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Argentina Molecular Diagnostics Market, By Product
- Latin America Molecular Diagnostics Market, By Product
- MEA
- MEA Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- MEA Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- MEA Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- MEA Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- South Africa
- South Africa Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- South Africa Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- South Africa Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- South Africa Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- South Africa Molecular Diagnostics Market, By Product
- Jordan
- Jordan Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Jordan Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Jordan Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Jordan Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Jordan Molecular Diagnostics Market, By Product
- UAE
- UAE Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- UAE Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- UAE Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- UAE Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- UAE Molecular Diagnostics Market, By Product
- Qatar
- Qatar Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Qatar Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Qatar Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Qatar Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Qatar Molecular Diagnostics Market, By Product
- Kuwait
- Kuwait Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Kuwait Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Kuwait Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Kuwait Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Kuwait Molecular Diagnostics Market, By Product
- Egypt
- Egypt Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
- Egypt Molecular Diagnostics Market, By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- Multiplex PCR
- Other PCR
- PCR, by Product
- Instruments
- Reagents
- Others
- PCR, by Type
- In Situ Hybridization (ISH)
- Instruments
- Reagents
- Others
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Others
- Chips and Microarrays
- Instruments
- Reagents
- Others
- Mass Spectrometry
- Instruments
- Reagents
- Others
- Transcription Mediated Amplification (TMA)
- Instruments
- Reagents
- Others
- Others
- Instruments
- Reagents
- Others
- Polymerase chain reaction (PCR)
- Egypt Molecular Diagnostics Market, By Application
- Oncology
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Blood Cancer
- Lung Cancer
- Other Cancer
- Pharmacogenomics
- Infectious Diseases
- Methicillin-resistant Staphylococcus Aureus (MRSA)
- Clostridium Difficile
- Vancomycin-resistant Enterococci (VRE)
- Carbapenem-resistant Bacteria
- Flu
- Respiratory Syncytial Virus (RSV)
- Candida
- Tuberculosis and Drug-resistant TBA
- Meningitis
- Gastrointestinal Panel Testing
- Chlamydia
- Gonorrhea
- HIV
- Hepatitis C
- Hepatitis B
- Other Infectious Disease
- Genetic Testing
- Newborn Screening
- Predictive and Presymptomatic Testing
- Other Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
- Oncology
- Egypt Molecular Diagnostics Market, By Test Location
- Point of care
- Central laboratories
- Self-test or Over the Counter
- Egypt Molecular Diagnostics Market, By Product
- MEA Molecular Diagnostics Market, By Product
- North America
Molecular Diagnostics Market Dynamics
Driver: Growth in global geriatric population
The geriatric population is growing rapidly across the globe. As per a UN report, as of 2020, around 727 million people aged 65 years and more were living worldwide. Moreover, the number of people of and above 80 years of age is expected to rise to 1.5 billion by 2050. Aging is a major risk factor for several diseases, which include diabetes and obesity; this sharply raises the risk of infectious diseases. The aging population is susceptible to diseases such as cardiovascular diseases, cancer, and neurological disorders also, which is expected to drive the demand for molecular diagnostics services.
According to the U.S. National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database, 43% of men and 38% of women are expected to be diagnosed with cancer in their lifetime. Around 67% of new cancer cases are diagnosed in people aged 65 years and more, highlighting the relation between aging and cancer. Molecular diagnostics has a significant impact on cancer management, as well as in managing infectious and cardiovascular disorders. This makes it critical for public health surveillance and detection.
Driver: Introduction of innovative and technologically advanced products
Rapid technological advancements that can lead to accurate results, as well as portability and cost-effectiveness, are major drivers for market expansion. Companies are upgrading their products by implementing new techniques to gain specific and accurate results. Companies are developing a range of molecular diagnostic techniques, such as Loop-Mediated Isothermal Amplification (LAMP) and Transcription-Mediated Amplification (TMA), for diagnosing tumors. Research using Next-Generation Sequencing (NGS) is a prominent application segment in the molecular diagnostics space and is extensively used in R&D in this field. Thermo Fisher Scientific launched the 5500x1 genetic analyzer and Illumina, Inc. introduced NextSeq CN500. These launches are expected to drive market growth, due to them being able to rapidly and accurately analyze DNA and histone modifications. Moreover, growing use of multiplex PCR technologies and real-time PCR equipment to detect DNA methylation is expected to drive industry expansion during the forecast period.
Key market players are constantly involved in updating their range of testing options for qPCR instruments through R&D initiatives to develop kits that target emerging diseases or by entering into agreements with notable kit manufacturing companies. An example is the introduction of Cobas HPV test assay in Cobas 4800 by Roche Diagnostics. The introduction of Xpert assay for tuberculosis testing by Cepheid on its GeneXpert platform is another example. Such developments are anticipated to drive the global market for molecular diagnostics. Growing use of FISH and ELISA technologies is also expected to drive growth during the forecast period.
Restraint: Presence of an ambiguous regulatory network
The regulatory framework for approvals has constantly posed challenges for biotechnology, pharmaceutical, and medical technology industries. The possibility of patient risks has driven the U.S. FDA premarketing guidelines. The regulatory atmosphere is complex and the FDA is becoming ever more careful, as dependency on molecular diagnostics to make serious medical decisions is rising. The FDA also conducts post-market investigation of IVD products to ensure consistency between performance and claims. For instance, a heavy penalty was imposed on Nichols Institute, a subsidiary of Quest Diagnostics, due to its product failing to match its performance claims. Hence, stringent regulations associated with approval for molecular diagnostics are limiting market growth.
Moreover, growing approval of rival technologies such as immunoassays and others due to technological advancements, POC, & automation are poised to affect demand for molecular diagnostics over the forecast period. For instance, in regions such as sub-Saharan Africa, portable immunoassay technologies for diagnosing malaria, tuberculosis, West Nile virus, and other infectious diseases are vital to mitigate frequent disease outbreaks. In December 2022, BioMérieux announced the approval of its automated immunoassay system “Vidas Kube,” which can be used in critical care emergency settings for detecting infectious diseases, immunochemistry, and food pathogens. Such initiatives are anticipated to restrain market growth through 2030.
What Does This Report Include?
This section will provide insights into the contents included in this molecular diagnostics market report and help gain clarity on the structure of the report to assist readers in navigating smoothly.
Molecular diagnostics market qualitative analysis
-
Industry overview
-
Industry trends
-
Market drivers and restraints
-
Market size
-
Growth prospects
-
Porter’s analysis
-
PESTEL analysis
-
Key market opportunities prioritized
-
Competitive landscape
-
Company overview
-
Financial performance
-
Product benchmarking
-
Latest strategic developments
-
Molecular diagnostics market quantitative analysis
-
Market size, estimates, and forecast from 2018 to 2030
-
Market estimates and forecast for product segments up to 2030
-
Regional market size and forecast for product segments up to 2030
-
Market estimates and forecast for application segments up to 2030
-
Regional market size and forecast for application segments up to 2030
-
Company financial performance
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationResearch Methodology
Grand View Research employs a comprehensive and iterative research methodology focused on minimizing deviance in order to provide the most accurate estimates and forecast possible. The company utilizes a combination of bottom-up and top-down approaches for segmenting and estimating quantitative aspects of the market. In Addition, a recurring theme prevalent across all our research reports is data triangulation that looks market from three different perspectives. Critical elements of the methodology employed for all our studies include:
Preliminary data mining
Raw market data is obtained and collated on a broad front. Data is continuously filtered to ensure that only validated and authenticated sources are considered. In addition, data is also mined from a host of reports in our repository, as well as a number of reputed paid databases. For a comprehensive understanding of the market, it is essential to understand the complete value chain, and in order to facilitate this; we collect data from raw material suppliers, distributors as well as buyers.
Technical issues and trends are obtained from surveys, technical symposia, and trade journals. Technical data is also gathered from an intellectual property perspective, focusing on white space and freedom of movement. Industry dynamics with respect to drivers, restraints, pricing trends are also gathered. As a result, the material developed contains a wide range of original data that is then further cross-validated and authenticated with published sources.
Statistical model
Our market estimates and forecasts are derived through simulation models. A unique model is created customized for each study. Gathered information for market dynamics, technology landscape, application development, and pricing trends are fed into the model and analyzed simultaneously. These factors are studied on a comparative basis, and their impact over the forecast period is quantified with the help of correlation, regression, and time series analysis. Market forecasting is performed via a combination of economic tools, technological analysis, industry experience, and domain expertise.
Econometric models are generally used for short-term forecasting, while technological market models are used for long-term forecasting. These are based on an amalgamation of the technology landscape, regulatory frameworks, economic outlook, and business principles. A bottom-up approach to market estimation is preferred, with key regional markets analyzed as separate entities and integration of data to obtain global estimates. This is critical for a deep understanding of the industry as well as ensuring minimal errors. Some of the parameters considered for forecasting include:
• Market drivers and restraints, along with their current and expected impact
• Raw material scenario and supply v/s price trends
• Regulatory scenario and expected developments
• Current capacity and expected capacity additions up to 2030We assign weights to these parameters and quantify their market impact using weighted average analysis, to derive an expected market growth rate.
Primary validation
This is the final step in estimating and forecasting for our reports. Exhaustive primary interviews are conducted, face to face as well as over the phone to validate our findings and assumptions used to obtain them. Interviewees are approached from leading companies across the value chain including suppliers, technology providers, domain experts, and buyers so as to ensure a holistic and unbiased picture of the market. These interviews are conducted across the globe, with language barriers overcome with the aid of local staff and interpreters. Primary interviews not only help in data validation but also provide critical insights into the market, current business scenario, and future expectations and enhance the quality of our reports. All our estimates and forecast are verified through exhaustive primary research with Key Industry Participants (KIPs) which typically include:
• Market-leading companies
• Raw material suppliers
• Product distributors
• BuyersThe key objectives of primary research are as follows:
• To validate our data in terms of accuracy and acceptability
• To gain an insight in to the current market and future expectationsData Collection Matrix
Perspective
Primary research
Secondary research
Supply-side
- Manufacturers
- Technology distributors and wholesalers
- Company reports and publications
- Government publications
- Independent investigations
- Economic and demographic data
Demand-side
- End-user surveys
- Consumer surveys
- Mystery shopping
- Case studies
- Reference customers
Industry Analysis MatrixQualitative analysis
Quantitative analysis
- Industry landscape and trends
- Market dynamics and key issues
- Technology landscape
- Market opportunities
- Porter’s analysis and PESTEL analysis
- Competitive landscape and component benchmarking
- Policy and regulatory scenario
- Market revenue estimates and forecast up to 2030
- Market revenue estimates and forecasts up to 2030, by technology
- Market revenue estimates and forecasts up to 2030, by application
- Market revenue estimates and forecasts up to 2030, by type
- Market revenue estimates and forecasts up to 2030, by component
- Regional market revenue forecasts, by technology
- Regional market revenue forecasts, by application
- Regional market revenue forecasts, by type
- Regional market revenue forecasts, by component
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationShare this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





