- Home
- »
- Medical Devices
- »
-
Neurothrombectomy Devices Market Size Report, 2030GVR Report cover
![Neurothrombectomy Devices Market Size, Share & Trends Report]()
Neurothrombectomy Devices Market Size, Share & Trends Analysis Report By Product (Clot Retrievers, Aspiration/ Suction Devices), By End-use (Hospitals, Emergency Clinics), By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-2-68038-941-8
- Number of Pages: 110
- Format: Electronic (PDF)
- Historical Range: 2018 - 2022
- Industry: Healthcare
Neurothrombectomy Devices Market Trends
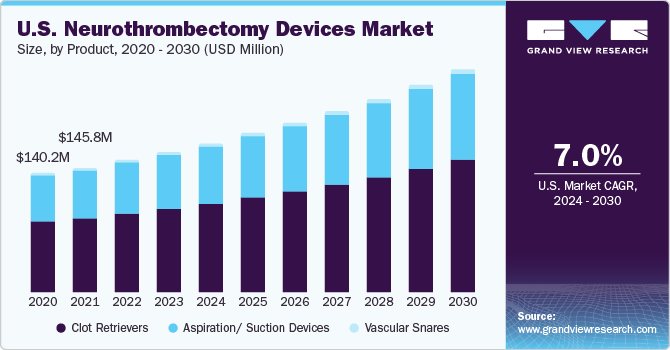
The global neurothrombectomy devices market size was estimated at USD 695.53 million in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.87% from 2024 to 2030. Increasing incidences of acute ischemic stroke globally are majorly driving the market growth. Furthermore, increased adoption of unhealthy lifestyles and increasing awareness of the disorder among the population is fueling market growth. According to WHO, stroke is the second most common cause of death, followed by ischemic heart disease. Stroke is generally classified into three categories: hemorrhagic stroke, ischemic stroke, and subarachnoid hemorrhage. Ischemic stroke occurs when a blood vessel is blocked, limiting the amount of blood that can reach the brain.
For instance, according to a report published by World Stroke Organization (WSO) in 2022, more than 62% of all stroke incidents are ischemic strokes. Out of these, 45% of ischemic strokes occurred in men and 55% in women. Approximately 3.3 million people die due to ischemic stroke annually. Furthermore, according to the CDC, in the U.S., there are more than 795,000 victims of stroke annually. Of these, about 610,000 of the victims are new or first strokes. Thus, the growing prevalence of ischemic stroke is expected to contribute to market growth.
Increasing awareness about neurological conditions such as stroke has resulted in people being mindful of the causes and early manifestations of stroke. Moreover, the FDA has been collaborating with neurological devices manufacturers and professional societies, such as the NeuroPoint Alliance and the National Institutes of Health (NIH) (StrokeNet), to help develop robust registries that can also be used to generate data for premarket application and postmarket surveillance.
An unhealthy lifestyle has doubled the risk of stroke among people. The most notable causes are unhealthy diet leading to obesity, physical inactivity, increased stress in work or life, and excessive consumption of alcohol & tobacco. Hypertension, cigarette smoking, and diabetes are recognized as primary risk factors for stroke. An unhealthy diet containing trans fats, cholesterol, and saturated fats may lead to stroke.
Stroke remains the leading cause of mortality and morbidity due to adoption of unhealthy lifestyle. For instance, according to CDC, one of the main factors contributing to major long-term disability is stroke. More than half of stroke survivors who are 65 years and older experience reduced mobility. Also, according to WHO, 15 million individuals suffer from stroke globally. Thus, increasing the adoption of unhealthy lifestyle is likely to boost market growth.
Furthermore, introduction of technologically advanced products and rapid product approval process are factors expected to fuel market growth over the forecast period. There are various other technologies used in the development of neurothrombectomy devices for the treatment of acute ischemic stroke (AIS) and its associated symptoms. For instance, in January 2023, Infinity Neuro announced CE Mark approval for its Inspira aspiration catheters. This is the company’s debut product; the company intends to follow up in 2023 and beyond with the release of a full line of products for the treatment of ischemic and hemorrhagic stroke.
End-use Insights
Based on end-use, the market is categorized into hospitals, ambulatory surgical centers, and emergency clinics segments. In 2023, the hospitals segment dominated the market with the largest revenue share of 71.25%. Hospitals are widely preferred by patients in comparison to emergency clinics owing to improved healthcare facilities and the availability of advanced equipment. Increasing incidence of acute ischemic diseases and growing awareness about neurothrombectomy devices are factors expected to boost segment growth. Stroke is the leading cause of death and disability in the U.S. & Europe. Reperfusion therapy using neurothrombectomy devices is the preferred treatment for AIS. Thus, the increasing prevalence of stroke is driving segment growth.
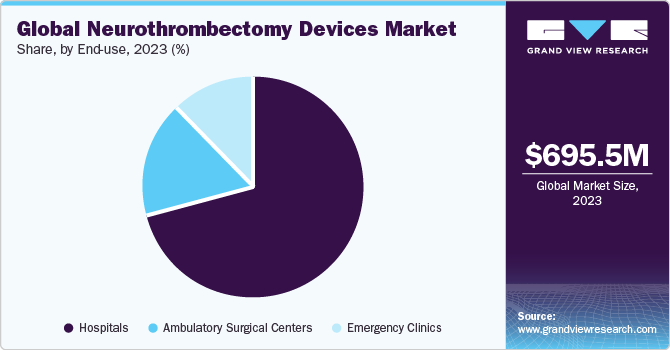
The ambulatory surgical centers (ASCs) segment is anticipated to witness the fastest CAGR of 7.7% over the forecast period. The shorter waiting time offered by ASCs to patients is expected to drive the segment at a significant rate during the forecast period. Furthermore, increasing demand for minimally invasive surgical procedures, introduction of technologically advanced products are expected to boost the market growth. For instance, in October 2021, Phenox GmbH introduced pRESET 6-50 mechanical thrombectomy device globally to develop its technology for AIS. The product has the longest stent retriever in the phenox product portfolio. Such advancements are expected to boost the segment growth over the forecast period.
Product Insights
Based on product, the market is categorized into aspiration/ suction devices, clot retrievers, and vascular snares segments. The clot retriever segment dominated the market in 2023 with the largest revenue share of 58.23%. Clot retriever devices are witnessing significant growth due to the rising prevalence of AIS and an increasing number of product launches by key market players. It is majorly used for removing blood clots from the cerebral arteries. Furthermore, the use of clot retrievers has increased in elderly patients (aged 55 to 65) due to growing incidence of stroke. Hence, growing cases of targeted disorder are propelling segment growth.
However, the aspiration/suction devices segment is anticipated to witness the fastest CAGR of 7.1% over the forecast period, owing to its increasing preference by physicians. In addition, these devices are witnessing growth due to a rise in the prevalence of neurological disorders among a large population. These devices vacuum clot out of the blood vessels and re-vascularize large vessel occlusion with accuracy. In addition, growing use of latest technology for treatment of stroke are factors increasing the overall growth of aspiration devices. For instance, in January 2023, the U.S. FDA approved and launched Lightning FlashTM, the most sophisticated and potent mechanically operated thrombectomy system available, according to Penumbra, Inc. Penumbra's groundbreaking Lightning Intelligent Aspiration technology, recently enhanced with double clot identification algorithms, is featured in Lightning Flash. The purpose of Lightning Flash is to rapidly remove big blood vessel clots in the body, such as venous thrombus and pulmonary emboli (PE), by combining cutting-edge catheter technology. Thus, ongoing product launches and regulatory approvals are expected to increase the adoption of aspiration devices.
Regional Insights
Europe dominated the market for neurothrombectomy devices with the largest revenue share of 32.74% in 2023 owing to the increasing number of patients suffering from acute ischemic stroke. Europe is one of the most advanced regions globally with innovative technologies and infrastructure resulting in significant healthcare facilities and patient care. In addition, an increase in investments by government & private institutions is also expected to drive the market growth in this region. Moreover, increasing approvals for neurothrombectomy devices and the launch of new products by manufacturers in the region are expected to boost the market growth. For instance, in April 2022, to evaluate the function of brain blood clot extraction devices in stroke patients, Medtronic established a clinical registry. To get actual data about the usage of revascularization devices in patients with AIS, the business established a registry. Up to 200 participants were enrolled in the registry, which was planned to run for about 2.5 years.
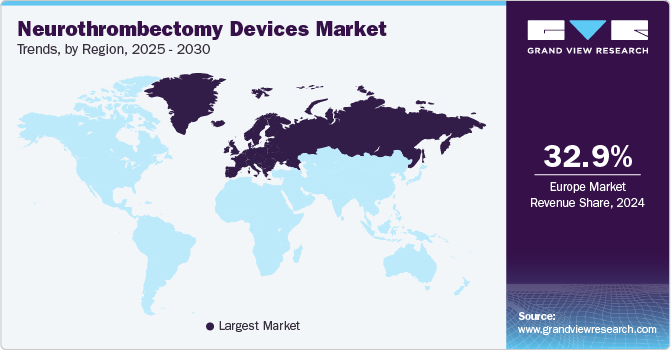
North America is anticipated to witness a significant CAGR over the forecast period. The growing population along with the increased risk of AIS disorder in the region is fueling the market significantly. In addition, technological advancements, presence of key participants, huge investments by governments for development of innovative medical devices, as well as growing demand for minimally invasive surgeries are driving the market growth. Also, presence of extensive public & private funding initiatives, especially in the U.S., to create awareness regarding advanced devices. In addition, an increase in the R&D investment by the manufacturers and a rise in the government initiatives are contributing immensely to regional market growth. For instance, recently in April 2021, the Society of Neurointerventional Surgery (SNIS) and NeuroPoint Alliance (NPA) collaborated with the Society of Vascular and Interventional Neurology (SVIN) to the NeuroVascular Quality Initiative-Quality Outcomes Database (NVQI-QOD).
Key Companies & Market Share Insights
The key players are focusing on growth strategies, such as new product launches, regulatory approvals, expansion, collaborations, acquisitions, and partnerships. For instance, in September 2023, the safety and effectiveness of Vesalio's NeVa thrombectomy platform in treating acute ischemic stroke were evaluated in the FDA-approved investigational product exclusion study CLEAR. The NeVa device is superior to other stent retrievers in terms of speed and efficacy of revascularization. The results of this study were released by Vesalio. This initiative was expected to boost the market for neurothrombectomy devices over the forecast period.
Key Neurothrombectomy Devices Companies:
- Medtronic
- Acandis GmbH
- Phenox GmbH
- Stryker
- Penumbra, Inc.
- Vesalio
Neurothrombectomy Devices Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 739.81 million
Revenue forecast in 2030
USD 1.10 billion
Growth Rate
CAGR of 6.87 % from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Report updated
November 2023
Quantitative units
Revenue in USD million and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Medtronic; Acandis GmbH; Phenox GmbH; Stryker; Penumbra, Inc.; Vesalio
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Neurothrombectomy Devices Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global neurothrombectomy devices market report based on product, end-use, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Clot Retrievers
-
Aspiration/Suction Devices
-
Vascular Snares
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospital
-
Emergency Clinics
-
Ambulatory Surgical Centers
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global neurothrombectomy devices market size was estimated at USD 695.53 million in 2023 and is expected to reach USD 739.81 million in 2024.
b. The global neurothrombectomy devices market is expected to grow at a compound annual growth rate of 6.87% from 2024 to 2030 to reach USD 1.10 billion by 2030.
b. Europe dominated the neurothrombectomy devices market with a share of 32.74% in 2023. This is attributable to the increasing number of patients suffering from acute ischemic stroke, increasing approvals for neurothrombectomy devices, and the launch of new products by manufacturers.
b. Some of the key players operating in the neurothrombectomy devices market include Medtronic, Stryker Corporation, Acandis GmbH, Stryker, Phenox GmbH, Penumbra Inc., Vesalio.
b. Key factors that are driving the neurothrombectomy devices market growth include the increasing prevalence of Acute Ischemic Stroke (AIS), rising adoption of an unhealthy lifestyle, and an increasing number of initiatives worldwide.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





