- Home
- »
- Pharmaceuticals
- »
-
Global Pediatric Vaccines Market Size, Industry Report, 2018-2025GVR Report cover
![Pediatric Vaccines Market Report]()
Pediatric Vaccines Market Analysis By Type (Monovalent, Multivalent), By Technology (Live Attenuated, Inactivated, Subunit, Toxoid, Conjugate), By Application (Infectious Diseases, Cancer, Allergy), By Region, And Segment Forecasts, 2018 - 2025
- Report ID: GVR-2-68038-279-2
- Number of Pages: 130
- Format: Electronic (PDF)
- Historical Range: 2014 - 2016
- Industry: Healthcare
Industry Insights
The global pediatric vaccines market size was estimated at USD 22.4 billion in 2016. An increase in government and non-government funding for the development of novel vaccines, along with initiatives for raising awareness about immunization, is likely to have a positive impact on market growth.
In an attempt to curb healthcare expenditure, governments have been making continuous efforts to reduce the cost of patient immunization through various vaccination programs funded by government and private health authorities. The Vaccines for Children Program, for instance, provides vaccines to children who lack health insurance or who cannot afford the cost of vaccination. Funding is provided through the Centers for Medicare and Medicaid Services (CMS) to the Centers for Disease Control (CDC) and approved by the Office of Management & Budget.
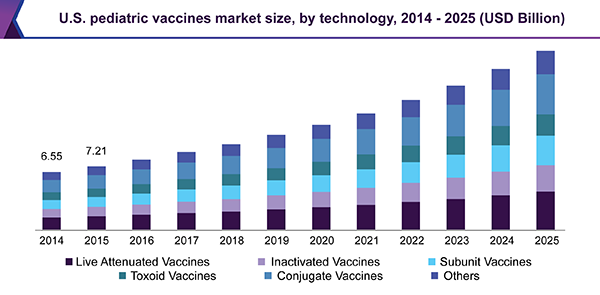
The rise in uptake of new vaccines has led to an increase in global vaccination coverage. According to WHO, immunization prevents around 3 million deaths worldwide every year, and an additional 1.5 million deaths could be avoided if vaccination coverage improves. According to a report released in 2015 by the Centers for Disease Control and Prevention (CDC), vaccination was estimated to prevent around 21 million hospitalizations, 322 million illnesses, and 732,000 deaths in children born between 1994 and 2013, which could save around USD 1.4 trillion in societal costs. By 2016, DTP vaccine coverage had reached approximately 90% in over 130 countries worldwide.
The high prevalence of chronic diseases is one of the major factors boosting the market for pediatric vaccines. According to WHO, around 5 billion severe illnesses and 0.5 billion annual deaths are reported globally. Infants are at a higher risk of contracting severe diseases, and therefore, vaccination plays an important role in their prevention. According to WHO, in 2015, around 134,200 people died due to measles, which accounted for nearly 15 deaths every hour. It also estimated that around 19.5 million children worldwide did not receive DTP vaccines in 2016.
Type & Technology Insights
The monovalent vaccines segment held the largest revenue share in the market for pediatric vaccines in 2016 owing to their safety and stability and their ability to develop a rapid immune response. The multivalent vaccines segment is expected to witness lucrative growth over the forecast period due to an increase in the prevalence of infectious diseases in children and the focus of major players on developing hexavalent, tetravalent, and pentavalent vaccines.
On the basis of technology, the market for pediatric vaccines is segmented into live attenuated, inactivated, subunit, toxoid, conjugate, and others. The conjugate vaccines segment dominated the market in 2016 and is likely to witness the fastest growth over the forecast period. This can be attributed to an increase in the prevalence of pneumococcal and meningococcal infections and a rise in awareness regarding the prevention of diseases.
Application Insights
On the basis of application, the market for pediatric vaccines is categorized into infectious diseases, cancer, and allergies. The infectious diseases segment holds the largest share due to growing global awareness about immunization against a number of infectious diseases that cause mortality and morbidity.
An increase in the incidence of diseases, such as chickenpox, typhoid, cholera, measles, and hepatitis, in children, results in high demand for immunization, which positively impacts the market.
Regional Insights
North America held the largest revenue share in the market for pediatric vaccines in 2016. Some of the key factors that can be attributed to its dominance are an increase in R&D investments by numerous companies and a rise in government support for immunization and the development of pediatric vaccines. In addition, the presence of advanced healthcare infrastructure and facilities for immunization supports the growth of this segment.
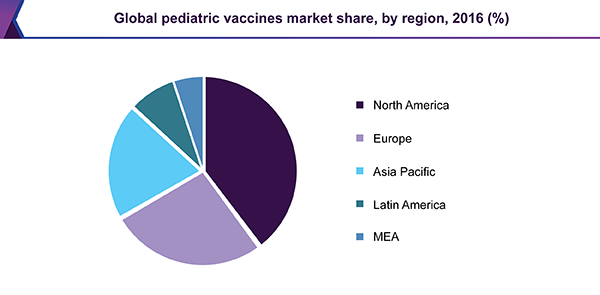
Asia Pacific is expected to witness the fastest growth over the forecast period due to an increase in the focus of major players on the Indian market for pediatric vaccines, a rise in immunization initiatives by government and non-government organizations, and the presence of a large target population. Asia Pacific is projected to expand at a significant CAGR of 12.6% over the forecast period.
Competitive Insights
Some of the major players dominating the market for pediatric vaccines are GlaxoSmithKline plc; Merck Sharp & Dohme Corp.; SANOFI; AstraZeneca; Pfizer, Inc.; Zydus Cadila; Indian Immunologicals Limited; Serum Institute of India Pvt. Ltd.; Mitsubishi Tanabe Pharma Corporation; Panacea Biotec; Sinovac Biotech Ltd.; and Bio Med Pvt. Ltd.
Key players mainly focus on expanding their product portfolio and invest in mergers and acquisitions to capture a larger revenue share. For instance, GlaxoSmithKline plc, in 2015, acquired the vaccine business division of Novartis and received approval for two new vaccines-Infanrix and Boostrix, which provide immunization against diphtheria, tetanus, and pertussis.
Report Scope
Attribute
Details
Base year for estimation
2016
Actual estimates/Historical data
2014 - 2016
Forecast period
2017 - 2025
Market representation
Revenue in USD billion and CAGR from 2017 to 2025
Regional scope
North America, Europe, Asia Pacific, Latin America, Middle East & Africa
Country scope
U.S., Canada, Germany, U.K., China, India, Japan, Brazil, Mexico, South Africa
Report coverage
Revenue forecast, company share, competitive landscape, growth factors, and trends
15% free customization scope (equivalent to 5-analyst working days)
If you need specific market information, which is not currently within the scope of the report, we will provide it to you as a part of customization
Segments covered in the reportThis report forecasts revenue growth at global, regional, and country levels and provides an analysis on industry trends in each of the sub-segments from 2014 to 2025. For the purpose of this study, Grand View Research, Inc. has segmented the global pediatric vaccines market on the basis of type, technology, application, and region:
-
Type Outlook (Revenue, USD Billion, 2014 - 2025)
-
Monovalent
-
Multivalent
-
-
Technology Outlook (Revenue, USD Billion, 2014 - 2025)
-
Live Attenuated
-
Inactivated
-
Subunit
-
Toxoid
-
Conjugate
-
Others
-
-
Application Outlook (Revenue, USD Billion, 2014 - 2025)
-
Infectious Disease
-
Cancer
-
Allergy
-
-
Regional Outlook (Revenue, USD Billion, 2014 - 2025)
-
North America
-
The U.S.
-
Canada
-
-
Europe
-
Germany
-
U.K.
-
-
Asia Pacific
-
China
-
Japan
-
India
-
-
Latin America
-
Brazil
-
Mexico
-
-
Middle East & Africa
-
South Africa
-
-
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





