- Home
- »
- Medical Devices
- »
-
Preterm Birth And PROM Testing Market Size Report, 2030GVR Report cover
![Preterm Birth And PROM Testing Market Size, Share & Trends Report]()
Preterm Birth And PROM Testing Market Size, Share & Trends Analysis Report By Test Type (Pelvic Exam, Ultrasound, Biochemical Markers, Uterine Monitoring), By Application (PROM, Preterm Labor), By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-3-68038-055-2
- Number of Pages: 120
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
The global preterm birth and PROM testing market size was valued at USD 1.30 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 8.3% from 2023 to 2030. Growing advancements in point-of-care diagnostics is also expected to have positive impact on the preterm birth and PROM testing market. Increasing average age of pregnant women leading to an exponential rise in the number of early births, delaying parenthood due to sociodemographic factors, including education/career goals, growing impact of women’s empowerment movements, and financial barriers is one of the key factors driving adoption of tests in the market.
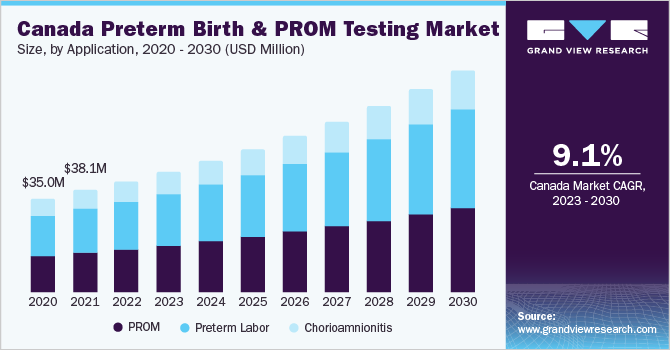
The outbreak of the COVID-19 pandemic and the high risk of PROM associated with COVID-19 infection has upsurged the demand for its testing solutions. According to the data published by Nebraska in March 2022, pregnant women with COVID-19 are at increased risk for critical complications and might be at high risk for preterm birth. Moreover, the disrupted global supply chain affected the movement of testing solutions across some countries, thus, having a considerable negative impact on the market.
According to a research conducted by group of researchers in Southern Ethiopia in December 2021, hypertension during pregnancy, history of either abortion, or cesarean section are the major causes of complications during the birth. The study recommended that early identification tests and treatment can minimize the risk of premature rupture of fetal membranes. Other factors that raise the probability of PROM in pregnant women are low socioeconomic conditions, sexually transmitted infections, and cigarette smoking during pregnancy. Hence, increasing the determinants of PROM is anticipated to positively affect the demand for PROM testing.
According to UNICEF, India accounts for 25.0 million births each year and witnesses death of one child every minute, with the causality rate due to premature birth at 35.0%. While substantial development in Special Newborn Care Units (SNCUs) has led to a decline in neonatal mortality; the socio-cultural barriers in health care for female infants are considered a major causality for female premature deaths. However, India has witnessed a significant reduction in the rate of child mortality, owing to the recent attention provided to the safety of female infants.
Governments and regulatory bodies are taking considerable measures to increase the accessibility of PROM testing in several regions. For instance, in March 2022, Sera Prognostics Inc. announced that the Centers for Medicare & Medicaid Services has set USD 750 as a Medicare payment rate for the company’s PreTRM test, a proteomic blood test to measure preterm birth risk.
Similarly, Actim PROM is a U.S. FDA-approved POC rapid test, to detect PROM. It provides rapid accurate results for preterm births. More than 7 million tests have been distributed in over 70 countries since it is highly reliable with 97% sensitivity and specificity. Actim Prom helps detect the protein biomarker Insulin-like Growth Factor Binding Protein-1 (IGFBP - 1). The advancements in POC diagnostics for PROM can encourage pregnant women to test for PROM more frequently when compared to a long procedure of testing held in hospitals and clinics.
Test Type Insights
In 2022, the ultrasound segment held 31.61% of the global preterm birth and PROM testing market. Ultrasound imaging is one of the traditional methods of testing premature labor, however, researchers are integrating the traditional method with advanced Artificial Intelligence (AI) to offer the optimal solution. For instance, in March 2022, Ultrasound AI Inc. announced receiving its first patent for software, Preterm AI. The product combines ultrasounds and AI to predict premature birth.
The PAMG-1 immunoassay segment is estimated to register highest CAGR during the forecast period. Placental Alpha Microglobulin 1 (PAMG-1) is a protein biomarker for PROM testing. The protein is present in amniotic fluid during pregnancy. When the amniotic sac surrounding the baby is intact, the concentration of PAMG-1 in vaginal fluid is very low (0.05–0.2 ng/mL). High levels of PAMG-1 (2,000–25,000 ng/mL) in vaginal discharge indicate membrane rupture. In April 2018, Qiagen’s PartoSure (cervicovaginal PAMG-1 test kit) received FDA approval for evaluating the risk of premature birth in expecting mothers with symptoms of preterm labor.
Application Insights
The preterm labor segment held 42.27% of market share in 2022 and is anticipated to have a similar trend in the forecasted period with the highest CAGR. As per the Lancet article published in September 2021, China is the second highest region with preterm births. It is estimated that from 2012 to 2018, there has been an average increase of 1.3% per year in preterm rate. While 29.5% of preterm births are accounted for spontaneous preterm labor, and 27.8% for preterm premature rupture of the fetal membranes.
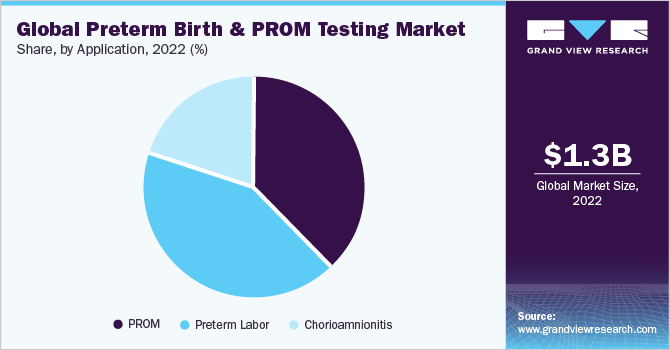
The Premature Rupture of Membranes (PROM) application segment is estimated to register considerable CAGR during the forecast period. As per data published in NCBI in June 2021, the PROM condition occurs in 8% to 10% of pregnancies worldwide. For accurate and timely management of PROM, it is important to emphasize efficient diagnosis. Some of the traditional diagnosis methods involve a vaginal examination, speculum examination, and fern tests, among others. However, the lower efficiency and correctness of these physical examinations lead to biomarker testing.
Regional Insights
North America captured the largest revenue share of 38.20% of preterm birth and PROM testing market in 2022 due to the changes in lifestyle factors such as excessive alcohol consumption & sedentary lifestyles, and high prevalence of preterm births in the region. Several awareness campaigns and initiatives being undertaken by nonprofit organizations in the region are expected to address challenges faced by premature babies and their families.
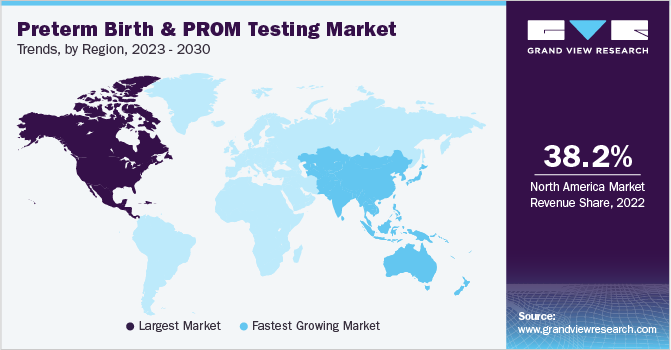
Asia Pacific is expected to expand at a fastest CAGR over the foreseeable future owing to the presence of a large population in India and China. Moreover, the rising number of preterm births in developing countries of the region is significantly creating a potential market in these regions, thereby leading to high growth of the market. Rapid technological advancements and increase in the number of awareness campaigns & nonprofit organizations supporting preterm infants and their families are some of the vital factors positively impacting the market. China is considered among the top five countries with highest preterm births and preterm birth is one of the major causes of neonatal death in India. The WHO reported 3.5 million premature births, and among these around 300,000 babies die due to associated complications.
Key Companies & Market Share Insights
The market is highly competitive with the presence of many international and local players. The major companies are focusing on strategies such as new product development, product enhancements, and regional expansion in an attempt to capture a large share of the market. For instance, in February 2023, Sera Prognostics announced positive results for its PreTRM Preterm Birth Test, a biomarker tests based on blood used for predicting the risk of preterm birth. The individuals were managed with the test-and-treat approach which resulted in reduction of neonatal death and decreased length of hospital stay. The positive results announcement also led to a spike in the company’s shares. Some of the prominent players of preterm birth and PROM testing market include:
-
Qiagen N.V.
-
Hologic, Inc.
-
Cooper Surgical Inc.
-
Abbott
-
Medixbiochemica
-
Sera Prognostics
-
Clinical Innovations, LLC
-
Biosynex
-
NX Prenatal, Inc.
-
IQ Products
Preterm Birth And PROM Testing Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 1.40 billion
Revenue forecast in 2030
USD 2.45 billion
Growth rate
CAGR of 8.3% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
July 2023
Quantitative units
Revenue in USD million/ billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Test type, application, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; U.K.; France; Italy; Spain; Denmark, Sweden, Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Qiagen N.V.; Hologic, Inc.; Cooper Surgical Inc.; Abbott; Medixbiochemica; Sera prognostics; Clinical Innovations; LLC, Biosynex; NX Prenatal; Inc., IQ Products
Customization scope
Free report customization (equivalent to up to 8 analyst's working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
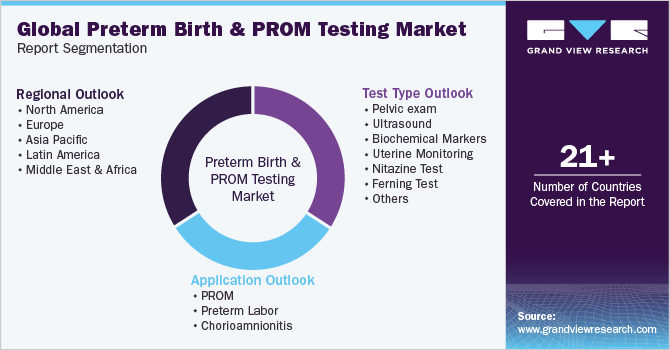
Global Preterm Birth And PROM Testing Market Report Segmentation
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented the global preterm birth and PROM testing market report on the basis of test type, application type, and region:
-
Test Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Pelvic exam
-
Ultrasound
-
Biochemical Markers
-
Interleukin (IL)-6
-
C-Reactive Protein (CRP)
-
IL-1, IL-2, IL-8, TNF-a
-
Corticotropin-Releasing Hormone (CRH)
-
Alpha-fetoprotein (AFP)
-
-
Uterine Monitoring
-
Nitazine Test
-
Ferning Test
-
Pooling
-
PAMG-1 Immunoassay
-
IGFBP Test
-
Fetal Fibronectin (fFN)
-
Others
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
PROM
-
Preterm Labor
-
Chorioamnionitis
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
U.K.
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. Some key players operating in the preterm birth and PROM testing market include Qiagen; Hologic; Cooper Surgical; Abbott Laboratories; Medixbiochemica; Sera Prognostics; Biosynex; and IQ Products.
b. Key factors that are driving the market growth include the growing incidence of preterm births, increasing geographical foothold of market players, high demand for effective preterm diagnosis, and improvement in point-of-care (POC) diagnostics.
b. The global preterm birth and PROM testing market size was estimated at USD 1.30 billion in 2022 and is expected to reach USD 1.40 billion in 2023.
b. The global preterm birth and PROM testing market is expected to grow at a compound annual growth rate of 8.3% from 2023 to 2030 to reach USD 2.45 billion by 2030.
b. North America dominated the preterm birth & PROM testing market with a share of 38.20% in 2022. This is attributable to the rising incidence of premature births, poor lifestyle choices, and increasing maternal age.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





