- Home
- »
- Medical Devices
- »
-
U.S. Long-acting Contraception Market Size Report, 2028GVR Report cover
![U.S. Long-acting Contraception Market Size, Share & Trends Report]()
U.S. Long-acting Contraception Market Size, Share & Trends Analysis Report By Product (Intrauterine Devices, Subdermal Implants, Injectables), And Segment Forecasts, 2021 - 2028
- Report ID: GVR-4-68039-680-4
- Number of Pages: 76
- Format: Electronic (PDF)
- Historical Range: 2016 - 2019
- Industry: Healthcare
Report Overview
The U.S. long-acting contraception market size was valued at around USD 1.7 billion in 2020 and is projected to grow at a compound annual growth rate (CAGR) of around 4.69% over the forecast period. The rising adoption of Intrauterine Devices (IUDs) owing to high effectiveness and increasing awareness about sexual health are expected to boost the use of various birth control methods. In recent years, the demand and usage of Long-Acting Reversible Contraception (LARC) have increased in the U.S. The use of LARCs was higher among women aged 30 to 39 years (12.7%) and 20 to 29 years (13.7%), as compared to women aged 40 to 49 years (6.6%) and 15 to 19 years (5.8%) during 2017 to 2019, as per the National Center for Health Statistics. Thus, higher adoption among middle-aged women is propelling the market growth.
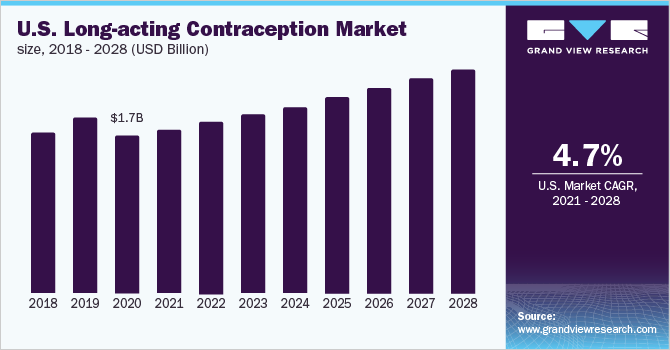
LARCs do not require ongoing effort from the patient, compared to short-term reversible contraceptive methods. Users are not required to remember to take pills daily, thus, removing the possibility of related errors. Furthermore, IUDs are not repetitive and can last for 3 to 5 years. IUDs are nearly 20 times more effective than oral pills, patches, and vaginal rings. These advantages over other modern and traditional contraceptive methods are the primary factors driving the adoption of LARC.
The American College of Obstetrics and Gynecologists regards LARCs, such as IUDs, as the gold standard in birth control. IUDs are one of the most effective birth control methods with over 99% effectiveness. According to Kaiser Family Foundation, IUD utilization among women aged 15 to 44 years, who have used contraceptives within the previous 30 days, increased from 12% in 2013-2015 to 14% in 2015-2017. This was primarily due to high effectiveness and ease of use.
Presently available LARCs are safe and long-lasting. They have high patient acceptability, fewer contraindications for use, and are recommended, in certain cases, due to their improved bleeding control. As the understanding and awareness about products, such as ParaGard & Kyleena, is growing among the U.S. population, their demand & popularity are witnessing substantial growth.
COVID-19 U.S. Long-acting Contraception Market Impact: 10.3% decline from 2019 to 2020
Pandemic Impact
Post COVID Outlook
During the initial period of 2020, the COVID-19-related restrictions were stringent and medical facilities were providing only necessary or urgent services while postponing elective procedures. This was one of the major factors that affected the in terms of reduced product sales
Relaxation in social distancing measures and reopening of medical facilities for non-urgent services in the country is likely to reverse the downtrend of product sales. For instance, sales of PARAGARD increased by 16% in the U.S. during the 1st quarter of 2021
Key market players reported a significant decline in their revenue due to decreased demand during the pandemic. For instance, Implanon sales declined by 14.1% in 2020 compared to 2019 in the U.S. While other manufacturers are facing the issue of reduced demand, Pfizer, Inc. announced to stop manufacturing of certain drugs including Depo-Provera at its Kalamazoo production site until early 2022
As clinics and hospitals are resuming services across the nation, backlogged procedures for contraceptive screening are anticipated to drive the product demand
The U.S. government is undertaking active efforts and initiatives to improve access to contraceptives. Affordable Care Act mandates full coverage of preventive services without any consumer sharing. Preventive services consist of prescription contraceptives and other related medical services. This has led to a considerable drop in out-of-pocket expenses, thereby improving accessibility. This is anticipated to fuel the market growth.
Product Insights
On the basis of product, the market is segmented into IUD, subdermal implants, and injectables. The IUD segment accounted for the largest market share of over 60.1% in 2020. High effectiveness in the prevention of pregnancy and improving access owing to the U.S. Affordable Care Act are primary factors responsible for the prominent share of the segment. The IUD segment is further bifurcated into hormonal and nonhormonal IUD products. The hormonal IUD segment accounted for the maximum share owing to improving awareness among women about reproductive health. In addition, recent advancements in available brands, such as Liletta, are expected to boost its adoption.
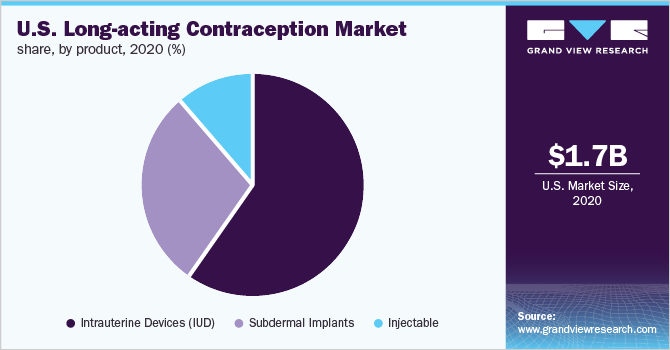
The subdermal implants segment is projected to witness the second-fastest CAGR during the forecast period. This growth is credited to the product’s cost efficiency, more than 99.0% effectiveness, and quick return of natural fertility after removal. A Contraceptive CHOICE study published in November 2017 reported higher acceptance of implants among the adolescent population. Thus, the high adoption of subdermal implants among teenagers is also likely to boost segment growth.
Key Companies & Market Share Insights
The market is consolidated with the presence of a few major players. Most of the companies are undertaking strategic initiatives, such as investments in R&D and the development of innovative products, to strengthen their market presence. The limited number of options for long-term contraceptives available in the market creates opportunities for companies to launch new products. In August 2021, the U.S. FDA approved a supplemental new drug application (sNDA) by Bayer AG, extending its IUD Mirena’s duration of use by 1 year, making the device available for use as contraception for up to 7 years. In October 2019, Allergan received the U.S. FDA approval for extending the use of Medicines360’s Supplemental NDA—LILETTA—for up to 6 years. Some of the prominent companies operating in the U.S. long-acting contraception market include:
-
Merck & Co., Inc.
-
Pfizer Inc.
-
The Cooper Companies Inc.
-
Allergan (AbbVie Inc.)
-
Bayer AG
U.S. Long-acting Contraception Market Report Scope
Report Attribute
Details
Market size value in 2021
USD 1.8 billion
Revenue forecast in 2028
USD 2.5 billion
Growth rate
CAGR of 4.69% from 2021 to 2028
Base year for estimation
2020
Historical data
2016 - 2019
Forecast period
2021 - 2028
Quantitative units
Revenue in USD million/billion and CAGR from 2021 to 2028
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product
Country scope
U.S.
Key companies profiled
Merck & Co., Inc.; Pfizer Inc.; The Cooper; Allergan (AbbVie Inc.); Bayer AG
Customization scope
Free report customization (equivalent to up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail of customized purchase options to meet your exact research needs. Explore purchase options
Segments Covered in the ReportThis report forecasts revenue growth at the country level and provides an analysis of the latest industry trends in each of the sub-segments from 2016 to 2028. For the purpose of this study, Grand View Research has segmented the U.S. long-acting contraception market report on the basis of product:
-
Product Outlook (Revenue, USD Million, 2016 - 2028)
-
Intrauterine Devices (IUD)
-
Hormonal IUD
-
Nonhormonal IUD
-
-
Subdermal Implants
-
Injectable
-
Frequently Asked Questions About This Report
b. The U.S. long-acting contraception market size was estimated at USD 1.7 billion in 2020 and is expected to reach USD 1.8 billion in 2021.
b. The U.S. long-acting contraception market is expected to grow at a compound annual growth rate of 4.69% from 2021 to 2028 to reach USD 2.5 billion by 2028.
b. Intrauterine Devices (IUD) dominated the U.S. long-acting contraception market with a share of 58.9% in 2020. This is attributable to increasing demand for hormonal IUDs, no adverse effects on breastfeeding, and normal fertility after removal of the device.
b. Some key players operating in the U.S. long-acting contraception market include Merck & Co., Inc.; Bayer AG; Allergan (AbbVie); Pfizer, Inc.; and; The Cooper Companies Inc.
b. Key factors that are driving the U.S. long-acting contraception market growth include increasing adoption of long-acting contraceptives, strong government support, and rising publicly funded family planning services.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





