Cardiac Resynchronization Therapy (CRT) Market Future Outlook: Smaller, Internal Placement and Enhanced Battery Life of Devices
The Cardiac Resynchronization Therapy (CRT) market has been in existence since 2001 when the first device was introduced in August by Medtronic Plc. This product “InSync,” was launched after it showcased substantial improvement in exercising capacity of patients, Peak Volume Oxygen Consumption (PVO2), and the reduction of QRS duration recorded during the Electrocardiograph (ECG) . This system comprises of leads/wires, a computer, and a battery enclosed in a metal case.
The pulse generator of the system weighed about 85 gm and underwent trials until April 2011, when it displayed promising results in terms of enhancing the standard of life for patients suffering from cardiac myopathies and heart failure.
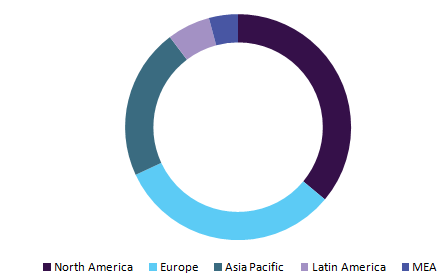
Global CRT market, by region, 2015
Following this, various such systems were launched and the U.S. FDA provided approvals to more than dozens of these products. The amalgamation of Implantable Cardioverter Defibrillators (ICD) with CRT pacemakers was further studied and formed the second-generation of the products. The Cardiac Resynchronization Therapy Defibrillators (CRT-D) devices are said to reduce the mortality rate in comparison to the sole use of CRT pacemakers.
As of 2015, the top contenders of the market space are working toward the development of the third-generation products, highlighted to have features such as:
- Wireless connectivity
- Internal placement of the pulse generator
- Prolonged battery life
The aforementioned attributes are expected to provide:
- Enhanced performance owing to pacing from within the heart chambers
- Reduced number of readmissions at the hospital
- Reduced cost
- Elongation of the replacement period to over 10 years
The wireless connectivity is expected to benefit the market by catering to currently non-responding heart failure patients, thereby increasing the target population and boosting the demand.
However, a device equipped with all the aforementioned features is still in its nascent stage of development and may become commercially viable post 2019. If these products are introduced over the forecast period, it may lead to an exponential growth of the industry.
A few advancements currently being explored by the market players are:
- LivaNova, in 2016, gained FDA approval for its third-generation PLATINUM CRT-D and ICD devices with a projected shelf life of 10 to 13 years.
- Research work is being performed by EBR Systems post the approval by the FDA for their Wireless Stimulation Endocardially (WiSE) technology. As of 2015, this technology is being used in the European region, but it will be investigated within the U.S. territory. This technology is closest in terms of the aforementioned features and would facilitate pacing by placement within the left ventricle of the heart.
- Besides advancing the existing technology, players such as Medtronic are introducing improved versions of the commercial devices. These products are expected to allow patients to undergo physical examination using techniques such as MRI without experiencing any complication. In 2016, the company launched two such products, Complia MRI Quad CRT-D SureScan and Amplia MRI Quad CRT-D SureScan. These systems also provide the benefit of reduction in readmission rate owing to their AdaptivCRT algorithm that minimizes the atrial fibrillation risk by about 46.0%.
Thus, rigorous R&D activities and routine introduction of enhanced products are expected to expedite the development and growth of the cardiac resynchronization therapy market over the forecast period.
 In-depth report on global cardiac resynchronization therapy market by Grand View Research:
In-depth report on global cardiac resynchronization therapy market by Grand View Research:
http://www.grandviewresearch.com/industry-analysis/cardiac-resynchronization-therapy-devices-market
To schedule a free market intelligence database demo, please complete the form below:
Service Guarantee
-
Insured Buying
This report has a service guarantee. We stand by our report quality.
-
Confidentiality
Your transaction & personal information is safe and secure.
-
Custom research service
Design an exclusive study to serve your research needs.
-
24/5 Research support
Get your queries resolved from an industry expert.