Pharmaceutical Analytical Testing Outsourcing Market Outlook: Analyst Perspective
Pharmaceutical analytical testing outsourcing services market is driven by innovation in pharmaceutical industry, increasing focus on regulatory, safety & quality, end-user volume, and pricing. Various categories of tests which are outsourced include bioanalytical tests, method development & validation, and stability testing.
Bioanalytical testing involves the examination of drugs/formulation or active ingredients within a biological system such as blood, urine, serum, tissue, and others. This analysis is performed in order to characterize and quantify the drug activity and provide evidence for a therapeutic action. These tests also help in creation of the drug monograph for reference during preclinical and clinical trial testing. Broadly, the segment is further classified into clinical and non-clinical bioanalytical testing and is expected to witness lucrative growth over the forecast period.
Global pharmaceutical analytical testing outsourcing market, by region, 2015
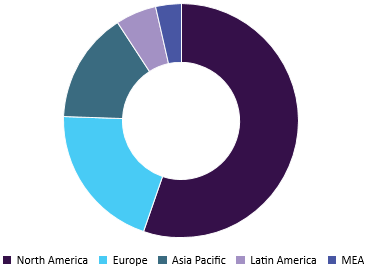
An increasing number of clinical trial registrations and entry of new participants within the marketspace since the past ten years has contributed to the growth of the bioanalytical testing needs. These tests are solely focused on understanding the quantitative aspects of the drug discovery and development process.
Besides offering the analytical services, vendors also perform method development and validation services in order to provide pharmaceutical firms with a complete package and a hassle-free experience. The analytical tests are crucial for introduction of the product for next phase of research. Hence, their method development & validation is expected to generate higher reliability and maintain proof of the results. With rising growth of outsourcing of testing processes, an ancillary growth may be witnessed for this service too, over the forecast period.
Asia Pacific is the fastest growing market owing to many developed countries investing in Asia Pacific regions and owing to the various amendments made by the regulatory market to change the clinical trials evaluation standards according to the global platform. In October 2014, the Australian service provider Novotech started a new office in China, its ninth office in Asia. Novotech, as a company, has expanded in many developing nations owing to the request by its U.S and EU clients. In addition, in February 2014, the Charles River Laboratories declared China as a country having disproportionate growth and expressed interest in merging with potential regions in the country.
In August 2015, WuXi Pharma Tech signed a strategic partnership with Lee Pharmaceutical for laboratory testing. The CRO will provide chemistry analytical services, oncology, in-vivo and in-vitro biology, immunology, pharmacokinetic/pharmacodynamics, in-vitro ADME, clinical bioanalysis, and toxicology for its R&D programs. Moreover, the Government of India has realized the potential in the CRO sector and has laid some action plans for boosting the CRO business India.
India is one of the favored destinations for outsourcing as it has many advantages such as trained manpower, reduced cost, time difference, and IT superpower. It is estimated that by 2020, India would be among the top healthcare economies in the world. Contract research and manufacturing outsourcing are the major factors driving the Indian outsourcing market. Availability of skill and expertise, low cost service models, increasing CROs, large patient pool are the advantage India possesses which is attracting the healthcare research outsourcing market.
 In-depth report on global pharmaceutical analytical testing outsourcing market by Grand View Research:
In-depth report on global pharmaceutical analytical testing outsourcing market by Grand View Research:
To schedule a free market intelligence database demo, please complete the form below:
Service Guarantee
-
Insured Buying
This report has a service guarantee. We stand by our report quality.
-
Confidentiality
Your transaction & personal information is safe and secure.
-
Custom research service
Design an exclusive study to serve your research needs.
-
24/5 Research support
Get your queries resolved from an industry expert.