- Home
- »
- Healthcare IT
- »
-
Pharmacovigilance And Drug Safety Software Market Size Report, 2030GVR Report cover
![Pharmacovigilance And Drug Safety Software Market Size, Share & Trends Report]()
Pharmacovigilance And Drug Safety Software Market Size, Share & Trends Analysis Report By Functionality, By Delivery Mode (On Premise, On Demand), By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-1-68038-144-3
- Number of Pages: 130
- Format: Electronic (PDF)
- Historical Range: 2017 - 2021
- Industry: Healthcare
Report Overview
The global pharmacovigilance and drug safety software market size was valued at USD 186.31 million in 2022 and is estimated to grow at a compound annual growth rate (CAGR) of 6.8% from 2023 to 2030. The growing availability of data, the need to process & derive insights from the generated data, stringent reporting norms & standards, and increasing software upgrades by key companies are some of the key drivers of this market. INTIENT Pharmacovigilance by Accenture is a comprehensive product suite that helps in the collection and management of a full spectrum of pharmacovigilance data. The platform also enables users to understand potential compliance issues, and emerging trends, and report on adverse events.
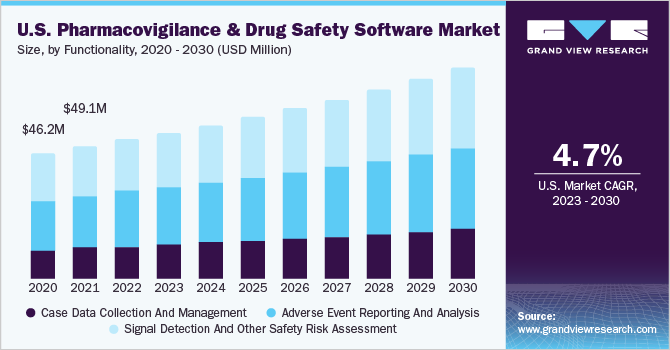
The growing demand of end-users for seamless connections between disparate data in an easy-to-use front end is a key driver contributing to the growth of the market for pharmacovigilance drug safety software. Customers are demonstrating an increasing preference for the analytical processing of drug safety data in an agile and flexible format. PV and drug safety software companies are thus offering machine learning, AI, data science, and other additions as part of their pharmacovigilance offering. Rapid drug development propelled by the COVID-19 pandemic has led to newer drugs being introduced at a rapid pace in the market at a faster rate. With the increase in the rate of drug consumption, the role of pharmacovigilance has emerged as critical in managing large quantities of data to assess the risks and benefits of a particular drug.
Increasing incidence of adverse drug reactions (ADR) and stringent regulations for drug development and approvals is expected to propel the demand for pharmacovigilance (PV) and drug safety software in the coming years. The presence of numerous market players has led to more and more PV and drug safety software solutions available in the market catering to varied customer requirements. Increasing globalization in pharmacovigilance with the widespread availability of the Internet is expected to boost usage rates. In addition, a growing health information functionality market will strengthen the usage of PV software over the forecast period, as it improves patient health and reduces medical expenditures.
Moreover, increasing pressure to follow safety guidelines set by government authorities such as the European Medicines Agency and the U.S. FDA is contributing to the growing adoption of pharmacovigilance systems. Many pharmaceutical companies are moving toward outsourcing pharmacovigilance activities to curb operational costs. Manufacturers are gradually shifting from being fully integrated pharmaceutical companies to sharing costs by collaborating with service providers. Services outsourced range from medical writing and clinical trial data collection to medical reporting and other PV services. Outsourcing helps increase internal resource flexibility, improves timelines, and results in better outcomes.
Functionality Insights
The signal management segment dominated the market for pharmacovigilance drug safety software and accounted for the largest revenue share of 35.0% in 2022. The case processing segment is estimated to grow the fastest in the coming years. This is due to the increasing need to avert errors in database management. These solutions are used to track individual case safety reports and avoid data redundancy through the elimination of errors. Key companies offer integrated end-to-end solutions to enhance their offerings and increase market share.
The case processing segment held a significant share in the market for pharmacovigilance drug safety software in 2022 owing to ADR reporting tools such as data entry and case management being increasingly used to comply with regulatory standards. Pharmacovigilance software has transformed the traditional way of ADR reporting and management. The introduction of advanced data integration solutions and the cost-effective nature of this PV system is anticipated to spur demand.
Delivery Mode Insights
On Demand software segment dominated the market and accounted for a largest revenue share in 2022 and segment held a significant share in the market for pharmacovigilance drug safety software in 2022. The introduction of cloud computing and its rapid adoption by healthcare IT providers is expected to propel the demand for the on-demand segment over the forecast period. On-demand solutions such as cloud-based S-a-a-S solutions are expected to gain popularity in the coming years. Increasing adoption of these platforms by pharmaceutical companies and contract research organizations is driving the segment. Remote access to data, real-time data tracking, and scalability are notable benefits associated with cloud-based systems.
End-use Insights
The pharma and biotech companies segment dominated the market and accounted for the largest revenue share of 35.0% in 2022. PV and drug safety software solutions are widely accepted by pharma and biotech companies to facilitate clinical trial programs and reduce the burden on medical expenditure. The segment thus dominated the market for pharmacovigilance drug safety software in 2021. Pharmacovigilance outsourcing is a growing trend in the pharmaceutical industry and as a result, manufacturers are striving to identify various ways to contain costs and minimize operational expenses by gradually shifting from being fully-integrated pharmaceutical companies to sharing costs through collaborations with service providers. This is expected to strengthen the growth of contract research firms that perform these activities for pharmaceutical companies.
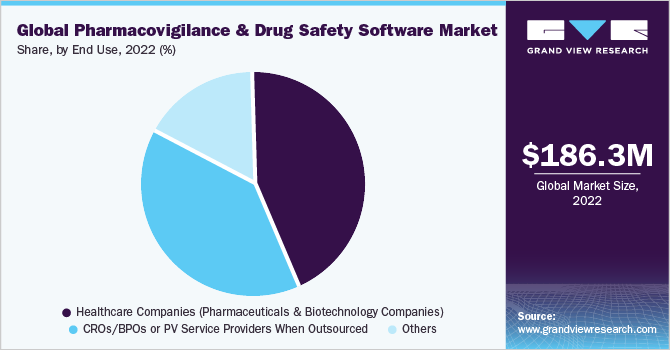
The CROs segment is expected to exhibit profitable growth during the forecast period. PV service providers, in an attempt to ensure sustainability, are providing customized end-to-end solutions to meet consumer needs. These firms are also incorporating integrated technologies, such as electronic data capture and hosting of PV warehousing to aggregate cross-industry data, which enables risk evaluation.
Regional Insights
North America led the market for pharmacovigilance drug safety software and accounted for the largest revenue share of 30.0% in 2022. The segment is anticipated to continue this trend over the foreseeable future. Owing to government-aided initiatives favoring the adoption of PV and drug safety software systems, the regional market for pharmacovigilance drug safety software is expected to show significant growth through 2030. For instance, the Open FDA initiative provides scientists and application developers access to its massive database through open search-based programs, which is anticipated to boost usage rates over the forecast period. Mini-Sentinel is a project started by the U.S. FDA to promote active surveillance systems, which provide statistically relevant data in lesser time. Initiatives such as these strengthen the growth of the regional market.
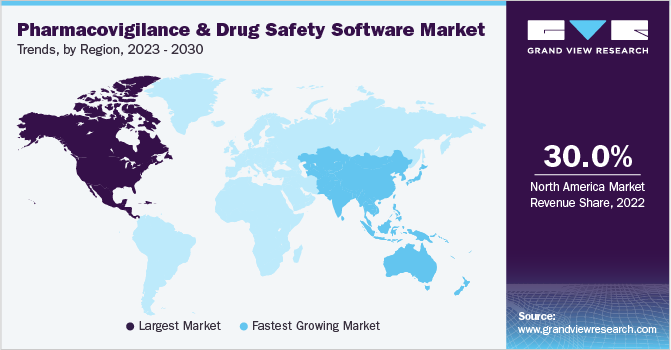
Asia Pacific is likely to experience the fastest CAGR during the forecast period. Companies in the Asia Pacific provide substantial cost-saving advantages, with savings ranging from 50% to 80% of the cost as compared to developed nations. This leads to a rising volume of clinical trials being conducted in this region. The rise in demand for and occurrence of clinical trials has led to an increasing focus on pharmacovigilance and drug safety software. Along with India and China, Singapore, South Korea, and Taiwan have been recognized as outsourcing hubs in Asia. The increasing number of adverse drug reactions, rising awareness about public safety, and stringent government regulations are some factors expected to drive market growth.
Key Companies & Market Share Insights
The market for pharmacovigilance drug safety software is competitive owing to the presence of many healthcare information technology companies, such as IBM, Oracle, and Cognizant. Key market players have also established separate clinical research data management and pharmacovigilance divisions to boost their market position and support their growth objectives. The introduction of technologically advanced and user-friendly software systems such as cloud-based pharmacovigilance and drug safety platforms is anticipated to drive market growth in the coming years. The presence of local vendors and new entrants is expected to lead to intense competition in the market for pharmacovigilance drug safety software in the coming years. For instance, Clinevo Safety by Clinevo Technologies is a cloud-based end-to-end Pharmacovigilance and Drug safety solution. It provides an all-in-one platform for Case Processing, Regulatory Submissions / AS2 Gateway, PV Intake, Analytics, and Safety Signals capabilities. Some of the prominent players in the Pharmacovigilance and drug safety software market include:
-
IQVIA
-
Accenture
-
Cognizant
-
Laboratory Corporation of America Holdings
-
IBM
-
ArisGlobal
-
ICON Plc.
-
Capgemini
-
Oracle
-
Parexel International Corporation
Pharmacovigilance And Drug Safety Software Market Report Scope
Report Attribute
Details
The market size value in 2023
USD 198.15 million
The revenue forecast in 2030
USD 314.11 million
Growth Rate
CAGR of 6.8% from 2023 to 2030
Base year for estimation
2022
Actual estimates/Historical data
2017 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Functionality, delivery mode, end-use, region
Regions covered
North America; Europe; Asia Pacific; Latin America; MEA
Country Scope
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Japan; China; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina, South Africa; Saudi Arabia, UAE, Kuwait
Key companies profiled
IQVIA; Accenture; Cognizant; Laboratory Corporation of America Holdings; IBM; ArisGlobal
ICON Plc.; Capgemini; Oracle; Parexel International Corporation
Customization scope
Free report customization (equivalent to up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail of customized purchase options to meet your exact research needs. Explore purchase options
Segments Covered in the ReportThis report forecasts revenue growth at the regional & country level and provides an analysis of the latest trends and opportunities in each of the sub-segments from 2017 to 2030. For this report, Grand View Research has segmented the global pharmacovigilance and drug safety software market report based on functionality, delivery mode, end-use, and region:
-
Functionality Outlook (Revenue, USD Million, 2017 - 2030)
-
Case data collection and management
-
Adverse event reporting and analysis
-
Signal detection and other safety risk assessment
-
-
Delivery Mode Outlook (Revenue, USD Million, 2017 - 2030)
-
On Premise
-
On Demand
-
-
End Use Outlook (Revenue, USD Million, 2017 - 2030)
-
Healthcare Companies (Pharmaceuticals & Biotechnology Companies)
-
CROs/BPOs or PV service providers when outsourced.
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2017 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Sweden
-
Denmark
-
Norway
-
-
Asia Pacific
-
China
-
India
-
Japan
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
MEA
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global pharmacovigilance and drug safety software market size was estimated at USD 186.31 million in 2022 and is expected to reach USD 198.15 million in 2023.
b. The global pharmacovigilance and drug safety software market is expected to grow at a compound annual growth rate of 6.8% from 2023 to 2030 to reach USD 314.11 million by 2030.
b. North America dominated the pharmacovigilance and drug safety software market in 2022. This is attributable to rising healthcare awareness coupled with cloud-based technologies acceptance and constant research and development initiatives.
b. Some key players operating in the pharmacovigilance and drug safety software market include IQVIA; Accenture; Cognizant; Laboratory Corporation of America Holdings; IBM; ArisGlobal; ICON Plc.; Capgemini; Oracle; and Parexel International Corporation.
b. Key factors that are driving the pharmacovigilance and drug safety software market growth include the increasing incidence of adverse drug reactions (ADR) and the demand for electronic reporting in pharmacovigilance.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





