- Home
- »
- Pharmaceuticals
- »
-
Specialty Generics Market Size, Share & Trends Report, 2030GVR Report cover
![Specialty Generics Market Size, Share & Trends Report]()
Specialty Generics Market Size, Share & Trends Analysis By Type (Injectables, Oral Drugs), By Application (Oncology, Inflammatory Conditions, Hepatitis C), By End-use (Specialty, Retail), By Region, and Segment Forecasts, 2023 - 2030
- Report ID: GVR-1-68038-629-5
- Number of Pages: 101
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
The global specialty generics market size was valued at USD 69.90 billion in 2022 and is expected to grow at a compounded annual growth rate (CAGR) of 9.9% from 2023 to 2030. Increasing adoption of specialty generic medicines for the treatment of complex chronic diseases such as hepatitis C and their increasing prevalence is a key driver for the market. According to World Health Organization (WHO) report 2022, globally around 58 million people have chronic hepatitis C virus infection, and about 1.5 million people are affected by this infection annually. Age is a major risk factor for rising prevalence of complex chronic diseases such as arthritis, cardiovascular diseases, and cancer due to weak immunity and high comorbid conditions.
According to the World Health Organization (WHO), the geriatric population increased from 1 billion in 2020 to 1.4 billion in 2021. By 2030, one in six people in the world will be aged 60 years and above. Hence, rapidly growing geriatric population is expected to create lucrative growth opportunities in the market. Major pharmaceutical companies have undertaken mergers & acquisitions, to gain a higher market share. In October 2021, BioCena acquired Pfizer-owned Australian plant for manufacturing drug therapies. In addition, in 2020, BioCena completed the acquisition of Pfizer Inc., owned by Hospira Adelaide. It is a leading supplier of 200 specialty generic injectables in the U.S. Such strategic initiatives are anticipated to drive market growth over the forecast period.
However, brand familiarity & loyalty, high complex nature of specialty generic products, and low profitability are some of the major entry barriers for new players in the market that lead to rising in drug prices. For instance, Teva Pharmaceutical Industries Ltd. offered smart co-pay cards to multiple sclerosis (MS) patients for buying COPAXONE for their treatment. Eligible card holders get drugs at zero cost. Such programs attract buyers attention to their branded products and thereby, negatively impact the sales of specialty generic products.
Type Insights
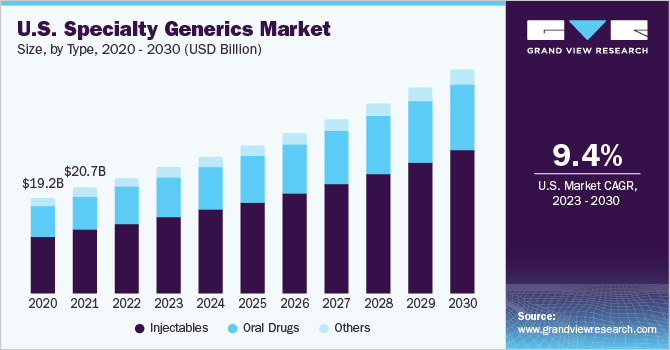
Based on type, the market is segmented into oral drugs, injectables, and others. The injectables segment held the highest share of 64.2% of the specialty generics market in 2022. The dominance of the injectables segment can be attributed to the benefits associated with injectables like long term effect, and immediate absorption, thus leading to higher patient compliance and acceptability.
Furthermore, injectables segment is also expected to grow at the fastest growth over the forecast period owing to increasing products approval and higher market penetration. For instance, in September 2021, Dr Reddy's Laboratories in partnership with Natco Pharma launched a generic version of Revlimid; Nat-lenalidomide & Reddy-lenalidomide approved by Health Canada for the treatment of patients with myelodysplastic syndrome and multiple myeloma in Canada. This approval provides easy access to low-cost specialty generic drugs to cancer patients, thereby, increasing prescription rate.
Application Insights
Based on application, the market is segmented into oncology, inflammatory conditions, multiple sclerosis, hepatitis C, and others. The inflammatory conditions segment dominated the market with a revenue share of 27.47% in 2022 due to increased prevalence of inflammatory conditions like rheumatoid arthritis. According to Global RA Network estimate, around 350 million people live with arthritis worldwide. In addition, according to the NHS, more than 10 million people are suffering from arthritis. Hence, the rise in prevalence of inflammatory conditions have paved the way for key players to enhance their market share in this sector.
Oncology segment is expected to witness the significant growth over the forecast period owing to rise in disease burden of cancer and ANDA product approvals such as generic Xtandi (Enzalutamide) for treating castration-resistant prostate cancer in May 2021. According to Globocan, the number of new cancer cases is anticipated to reach 28.4 million within the next two decades, with a rise of 47% from 2020, owing to adoption of western lifestyle, high consumption of alcohol, smoking, poor diet choices, and physical inactivity. Growing number of cancer cases is projected to propel the demand for specialty generics.
End Use Insights
On the basis of end-use, the market has been segmented into specialty pharmacy, retail pharmacy, and hospital pharmacy. The specialty pharmacy segment dominated the market with a revenue share of 76.87% in 2022 and is expected to witness lucrative growth over the forecast period. Major specialty generic manufacturers and insurance providers choose specialty pharmacies for the distribution of products due to negligible distribution costs and easy access to medicines. According to Drug Channels Institute’s report 2021, CVS specialty, Accredo, Optum specialty pharmacy, Walgreens stores, and Humana specialty pharmacy were world’s top five specialty pharmacies.
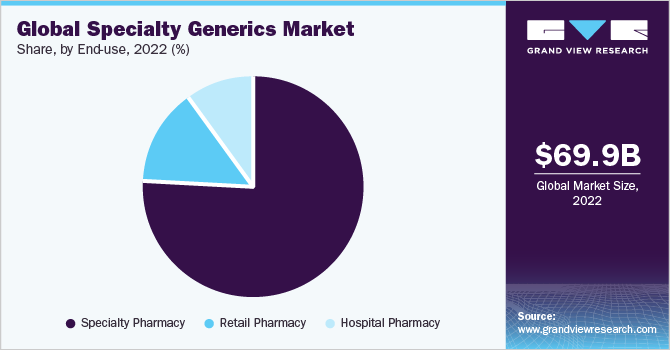
Furthermore, low cost associated with specialty generic drug inventory offers a great return on investment on medicines compared to branded products. Factors like timely delivery, optimizing patient access, and effective distribution management of specialty pharmacies attract buyers attention to specialty pharmacy and contributes in segment growth.
Regional Insights
North America dominated the market with a share of 36.3% in 2022 owing to the presence of supportive regulatory policies regarding the approval of new products. The U.S. FDA has undertaken several initiatives to smoothen overall approval process. Thus, the U.S. FDA introduced Generic Drug User Free Amendments (GDUFA) under the Hatch-Waxman act to quicken the delivery of safe and effective low cost generic drugs to the public. As a result, the key manufacturers are constantly making efforts for commercialization of specialty generic drugs in the market. For instance, in June 2022, Amneal Pharmaceuticals Inc., launched LYVISPAH (baclofen) a specialty product approved by U.S. FDA for the indication of multiple sclerosis and other spinal cord disorders.
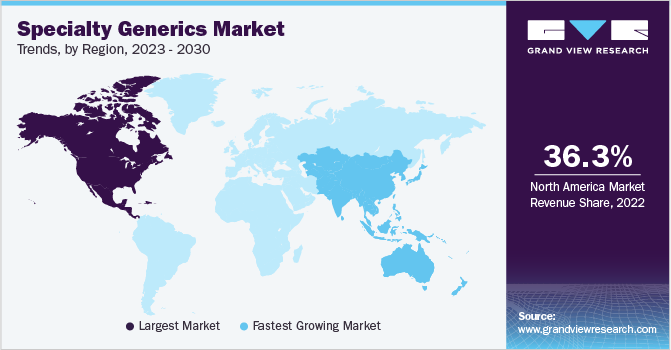
The Asia Pacific is estimated to be the fastest-growing region over the forecast period. The growth of region is mainly driven by high unmet low cost medicine need and launch of new generic drugs in region. In 2021, BDR Pharma launched generic version of Midostaurin with brand name of MSTARIN for the treatment of rare cancer like acute myeloid leukemia (AML).
Specialty Generics Market Share Insights
Regional expansion of product portfolio & service offerings is a key strategy adopted by players in the market. In June 2020, Sun Pharmaceutical Industries Ltd and Hikma Pharmaceuticals PLC signed an exclusive licensing and distribution agreement for Ilumya in the MEA region for the treatment of moderate to severe plaque psoriasis. This agreement offers a low-cost and equally effective specialty generic medicines to people with unmet treatment need. Some of the key players in the global specialty generics market include:
-
Teva Pharmaceuticals Industries Ltd
-
Viatris Inc.
-
Novartis AG (Sandoz International GmbH)
-
Hikma Pharmaceuticals PLC
-
Mallinckrodt
-
Bausch Health Companies Inc. (Valeant Pharmaceuticals International, Inc.)
-
Dr. Reddy’s Laboratories Ltd.
-
Endo Pharmaceuticals Inc.
-
Apotex Corp.
-
Sun Pharmaceutical Industries Ltd
-
Fresenius Kabi Brasil Ltda
-
STADA Arzneimittel AG
Specialty Generics Market Report Scope
Report Attribute
Details
Market size value in 2022
USD 77.52 billion
Revenue forecast in 2030
USD 148.72 billion
Growth rate
CAGR of 9.9% from 2022 to 2030
Base year for estimation
2023
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, application, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; U.K.; France; Italy; Spain; Denmark, Sweden, Norway, China; Japan; India; Australia; South Korea; Thailand, Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait.
Key companies profiled
Teva Pharmaceuticals Industries Ltd: Novartis AG (Sandoz International GmbH); Mallinckrodt; Bausch Health Companies Inc. (Valeant Pharmaceuticals International, Inc.); Endo Pharmaceuticals Inc.; Viatris Inc.;Fresenius Kabi Brasil Ltda; STADA Arzneimittel AG; Apotex Corp.; Hikma Pharmaceuticals PLC; Dr. Reddy’s Laboratories Ltd; Sun Pharmaceutical Industries Ltd.
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Specialty Generics Market Report Segmentation
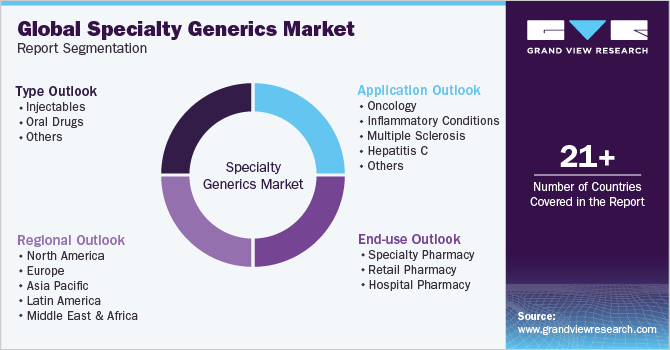
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented the specialty generics market on the basis of type, application, end-use, and regions:
-
Type Outlook (Revenue, USD Billion, 2018 - 2030)
-
Injectables
-
Oral drugs
-
Others
-
-
Application Outlook (Revenue, USD Billion, 2018 - 2030)
-
Oncology
-
Inflammatory conditions
-
Multiple sclerosis
-
Hepatitis C
-
Others
-
-
End Use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Specialty pharmacy
-
Retail pharmacy
-
Hospital pharmacy
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. Based on type, the injectables segment accounted for a share of 64.24% in 2022 due to high prescription and usage rate of injectables due to long-term effects and immediate absorption.
b. Some of the key players in the specialty generics market are Teva Pharmaceutical Industries Ltd; Sun Pharmaceutical Industries Ltd; Mallinckrodt; Viatris, Inc.; Fresenius Kabi Brasil Ltda; STADA Arzneimittel AG; and Novartis AG.
b. The major factors driving market growth are the rising number of off-patent specialty drugs, increasing demand for low-cost specialty generics drugs, increasing prevalence of chronic disease, and increasing emphasis to cut down healthcare expenditure.
b. The global specialty generics market size was valued at USD 69.9 billion in 2022 and is anticipated to reach USD 77.52 billion in 2023.
b. The global specialty generics market is expected to witness a compound annual growth rate of 9.9% from 2023 to 2030 to reach USD 148.72 billion by 2030.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





