- Home
- »
- Medical Devices
- »
-
Catheter Stabilization Devices Market Size, Share Report, 2030GVR Report cover
![Catheter Stabilization Devices Market Size, Share & Trends Report]()
Catheter Stabilization Devices Market Size, Share & Trends Analysis Report By Product (Arterial Securement, Central Venous Catheter Securement, Peripheral Securement, Urinary Securement), By End-use, By Region, And Segment Forecasts, 2022 - 2030
- Report ID: GVR-1-68038-642-4
- Number of Pages: 96
- Format: Electronic (PDF)
- Historical Range: 2017 - 2020
- Industry: Healthcare
Report Overview
The global catheter stabilization devices market size was valued at USD 1.2 billion in 2021 and is projected to grow at a compound annual growth rate (CAGR) of 7.3% during the forecast period 2022-2030. Factors such as increasing demand for minimally invasive surgeries; high prevalence of lifestyle diseases, such as cardiovascular diseases, renal failure, & cancers; increase in the number of surgeries that may require these devices for postoperative care; and an increase in need to reduce catheter-associated complications & infections are expected to drive the market. Population aging also contributes to an increased need for use of these devices. The World Health Organization (WHO) declared the COVID-19 to be an emergency health crisis. The healthcare industry had been badly impacted by the pandemic issue. Intubation was one of the most important procedures for handling COVID-19 patients.
The rise in the number of corona patients had increased the demand for ventilators, intubation, & ventilation stabilization equipment such as catheter stabilization devices. IVIG (Intravenous immunoglobin) was used as supportive therapy for COVID 19 patients. which adds to the increased demand for catheter stabilizing equipment to lessen the risks associated with catheter movement.
However, due to the relaxation of COVID-19 lockdown restrictions, the opening of production plants, and ongoing patient visits, the health service has resumed its normal pace. The Catheter Stabilization sector is expected to return to normal in the near future as a result of the recent introductions of Catheter Stabilization/Security devices.
The use of intravascular catheters is an integral part of modern healthcare across the globe. These have become an essential component in hospitals & clinics with a rise in their usage. 3M in one of its articles stated that hospitals and clinics purchase more than 150 million of these devices each year for the administration of medications, IV fluids, parenteral nutrition, and hemodynamic status monitoring. It also estimates that more than 5 million central intravascular catheters are placed every year. The prevalence of urinary diseases, such as cystitis, urinary retention, urinary incontinence, benign prostate hyperplasia, kidney stones, and other diseases & disorders that lead to bladder dysfunction, is constantly increasing. The National Association for Continence states that around 25 million people in the U.S. suffer from some form of incontinence. The demand for these devices for the management of patients suffering from urinary diseases is increasing. Thus, the growing use of these globally in various application areas is anticipated to ultimately contribute to the increasing usage of stabilization devices.
The Joint Commission in its monograph, “A Global Challenge, A Global Perspective”, states that an estimated 80,000 Central Line-Associated Bloodstream Infections (CLABSIs) occur in ICUs each year in the U.S. The number increases to 250,000 cases of CLABSIs each year if patients outside the IUs are included as well. An increase in cases of such infections is one of the key factors driving the need for their proper and effective securement. The CDC recommends the use of securement devices to reduce the risk of intravascular catheter-related infections. Australia and New Zealand Urological Nurses Society (ANZUNS) in its catheterization clinical guidelines recommends that the best practice for the management of indwelling catheters is the usage of a securement device.
Guidelines on securement are also issued by the National Institute for Health and Care Excellence (NICE), the U.K., and the Occupational Safety and Health Administration (OSHA), U.S. Presence of such guidelines are also anticipated to drive the usage of stabilization devices. However, catheters are an indispensable part of patient care, their growing usage increases the risk of patients contracting infections that may be minor or life-threatening. The prevalence of associated bloodstream infections is increasing owing to their wide use. Thus, their stabilization has become a fundamental aspect of patient care and plays an important part in reducing related complications. Treatment of these infections results in a rise in cost, an increase in the length of hospital stay, and an increase in patient morbidity.
Product Insights
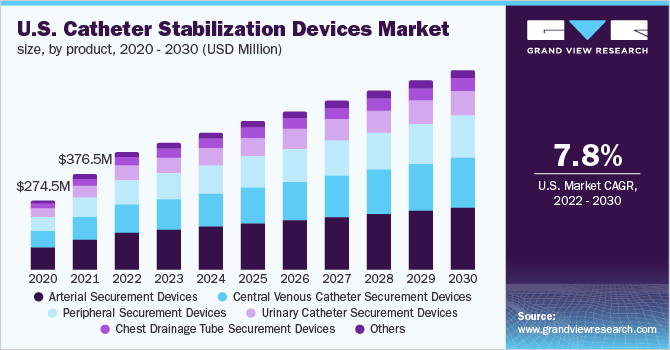
In 2021, the arterial securement devices segment held the largest share in the market. Arterial catheters are frequently used worldwide in routine modern healthcare practices. This segment held the majority of the market share owing to the extensive use of arterial catheters in a large number of surgeries associated with cardiovascular diseases and cancer therapies. The wide use of these devices makes securement important to reduce complications associated with their use.
Arterial catheters are widely used for interventions in ICUs globally. Rhode Island Medical Journal in its August 2014 issue stated that an estimated 6 million arterial catheters are placed in the U.S. each year. According to the Australian Vascular Access Society, up to 25% of arterial catheters fail during treatment owing to dislodgement, accidental removal, occlusion, or infection.
The CVC securement devices segment is expected to grow at the fastest rate with a CAGR of 8.1% during the forecast period. CVC securement is gaining importance owing to benefits such as the absence of interruptions during treatments, few repeat procedures & reinsertions, and a decrease in CVC replacement costs. An effective securement is crucial for critically ill patients requiring long-term therapy such as in the case of cancer.
End-use Insights
In 2021, the hospital segment held most of the share. A high number of surgeries are being performed in hospitals. Catheters have been widely used in hospitals for various kinds of treatments. The prevalence of infections associated with their usage is high in hospitalized patients. Catheter-Associated Urinary Tract Infections (CAUTI) are the most commonly reported hospital-acquired infections and their prevalence continues to increase.
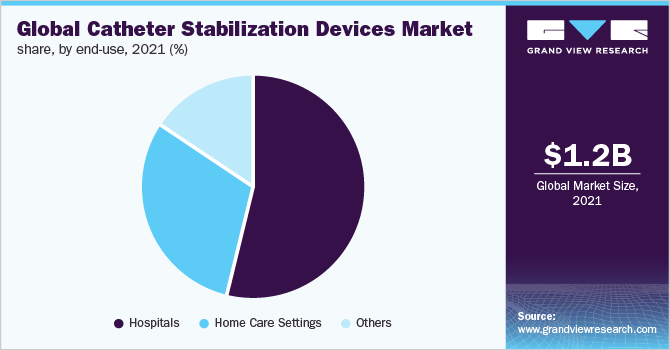
According to American Nurses Association (ANA), more than 560,000 patients acquire CAUTI every year. Hospital-acquired infections are one of the major threats to patient safety and the American Journal of Infection Control estimates that these infections cost around USD 33 billion. Thus, the growing prevalence of infections in hospitals contributes to the large share held by this segment.
The hospitals segment is also expected to continue its dominance with the fastest growth rate over the forecast period. Improving healthcare services in developing regions and the increasing number of hospitals globally is expected to contribute to the growing usage of stabilization devices.
Regional Insights
North America dominated the catheter securement devices market in 2021 with the highest revenue share. The large share of this region can be attributed to the high number of surgeries, which leads to an increase in the demand for stabilization devices The wide adoption of catheterization techniques in examination, diagnosis, and treatment is also one of the factors responsible for the market growth. Increasing usage of intravascular catheters in this region is further anticipated to drive the market.
The U.S. is the largest market in this region and across the globe due to the increase in the number of catheter placements. According to the Joint Commission, an estimated 300 million catheters are used each year in the U.S. of which nearly 3 million are central venous. High prevalence of CLABSIs and CAUTIs increases the need to curb these infections, which is anticipated to drive the market.
The Asia Pacific is expected to exhibit the fastest growth over the next few years owing to a large patient pool, growing target population, high unmet needs, and improving infrastructure in the region. Growing demand for various surgeries, improving healthcare facilities, and increasing awareness among the public and professionals are expected to boost growth in this region.
Key Companies & Market Share Insights
New product launches, expansion of product portfolio, and collaborations are crucial strategies employed by leading companies to capture a larger share of the global market.
In November 2021, CATHETRIX, an advanced manufacturer of urinary (Foley) smart catheter fixations, demonstrated their novel catheter stabilizer for preventing UTIs and unexpected Foley catheters extractions at MEDICA 2021 in Dusseldorf, Germany. Some of the prominent players operating in the global catheter stabilization devices market include:
-
C.R. Bard, Inc.
-
Baxter
-
3M
-
Centurion Medical Products
-
B Braun Melsungen AG
-
Merit Medical
-
Systems, Inc.
-
ConvaTec, Inc.
-
TIDI Products LLC
-
Smith’s Medical
Catheter Stabilization Devices Market Report Scope
Report Attribute
Details
Market size value in 2022
USD 1.5 billion
Revenue forecast in 2030
USD 2.4 billion
Growth rate
CAGR of 7.3% from 2022 to 2030
Base year for estimation
2021
Historical data
2017 - 2020
Forecast period
2022 - 2030
Quantitative units
Revenue in USD million and CAGR from 2022 to 2030
Report coverage
Revenue forecast, company share, competitive landscape, growth factors, and trends
Segment coverage
Product, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; Germany; U.K.; France; Spain; Italy; China; Japan, India, South Korea, Singapore, Australia, Brazil, Argentina, South Africa, UAE
Companies profiled
C.R. Bard, Inc.; Baxter; 3M; Centurion Medical Products; B Braun Melsungen AG; Merit Medical Systems, Inc.; ConvaTec, Inc.; TIDI Products, LLC; Smiths Medical
Customization scope
If you need specific market information, which is not currently within the scope of the report, we will provide it to you as a part of the customization
Pricing and purchase options
Avail of customized purchase options to meet your exact research needs. Explore purchase options
Segments Covered in the Report
This report forecasts revenue growth and provides an analysis of the industry trends in each of the sub-segments from 2017 to 2030. For this study, Grand View Research has segmented the global catheter stabilization devices market based on products, end-use, and region:
-
Products Outlook (Revenue, USD Million, 2017 - 2030)
-
Arterial Securement Devices
-
Central Venous Catheter Securement Devices
-
Peripheral Securement Devices
-
Urinary Catheter Securement Devices
-
Chest Drainage Tube Securement Devices
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2017 - 2030)
-
Hospitals
-
Home Care Settings
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2017 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
U.K.
-
-
Asia Pacific
-
Japan
-
China
-
-
Latin America
-
Brazil
-
Mexico
-
-
MEA
-
South Africa
-
-
Frequently Asked Questions About This Report
b. The global catheter stabilization devices market size was estimated at USD 1.2 billion in 2021 and is expected to reach USD 1.5 billion in 2022.
b. The global catheter stabilization devices market is expected to grow at a compound annual growth rate of 7.3% from 2022 to 2030 to reach USD 2.4 billion by 2030.
b. North America dominated the catheter stabilization devices market with a share of 36.3% in 2021. This is attributable to a high number of surgeries, and the wide adoption of catheterization techniques in examination, diagnosis, and treatment.
b. Some of the key players operating in the catheter stabilization devices market include C.R. Bard, Inc.(BD); Baxter; 3M, Centurion Medical Products; B Braun Melsungen AG; Merit Medical Systems, Inc.; ConvaTec, Inc.; TIDI Products, LLC; and Smiths Medical.
b. Key factors that are driving the catheter stabilization devices market growth include increasing usage of catheters and rising demand for minimally invasive surgeries.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





