- Home
- »
- Healthcare IT
- »
-
Clinical Trials Management System Market Size Report, 2030GVR Report cover
![Clinical Trials Management System Market Size, Share & Trends Report]()
Clinical Trials Management System Market (2024 - 2030) Size, Share & Trends Analysis Report By Solution Type, By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user, By Region, And Segment Forecasts
- Report ID: GVR-1-68038-150-4
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2023 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Clinical Trials Management System Market Summary
The global clinical trials management system market size was estimated at USD 1.85 billion in 2023 and is projected to reach USD 4.73 billion by 2030, growing at a CAGR of 14.64% from 2024 to 2030. The rapid growth of healthcare IT, along with a preference for decentralized clinical trials, initiatives by key industry players, and a rising number of clinical studies, are expected to fuel market growth.
Key Market Trends & Insights
- North America dominated the market with the revenue share of 50.01% in 2023.
- U.S. dominated the market in North America with the largest share of over 80.0% in 2023.
- Based on solution type, the enterprise segment led the market with the largest revenue share of 74.35% in 2023.
- Based on component, the software segment led the market with the largest revenue share of 56.04% in 2023.
- Based on delivery mode, the web & cloud-based segment led the market with the largest revenue share of 70.82% in 2023.
- Based on end-user, the CRO and other segments held the market with the largest revenue share of 40.10% in 2023.
Market Size & Forecast
- 2023 Market Size: USD 1.85 Billion
- 2030 Projected Market Size: USD 4.73 Billion
- CAGR (2024-2030): 14.64%
- North America: Largest market in 2023
- Asia Pacific: Fastest growing market
Government initiatives and investments by biotechnology and pharmaceutical firms are driving medical research activities. Combined with technological advancements, these factors are expected to propel market growth. For example, in October 2023, the Advanced Research Projects Agency for Health (ARPA-H), a U.S. Department of Health and Human Services (HHS) agency, announced plans to enhance the country's ability to conduct clinical trials rapidly, safely, and equitably. This initiative aims to promote technological advancements and insights to establish a robust national clinical trial infrastructure, thereby fostering the adoption of CTMS and strengthening the market growth.
Moreover, market players are introducing tailored solutions or optimizing existing ones to cater to the requirements of Decentralized Clinical Trials (DCTs). For instance, Cloudbyz offers a cloud-based CTM solution with features like ePRO, remote monitoring & SDV, eConsent, and eCRF, supporting virtual trials. Parexel International Corporation, a key player, has conducted over 250 fully virtual or hybrid DCTs and has experience with various remote patient engagement strategies incorporated into trials.
Increasing R&D investments by life science and medical device companies, coupled with the growing prevalence of acute & chronic disorders, are driving the surge in clinical trials. This trend is expected to boost the demand for solutions like CTMS, facilitating efficient management of diverse clinical trials and ultimately improving patient outcomes.
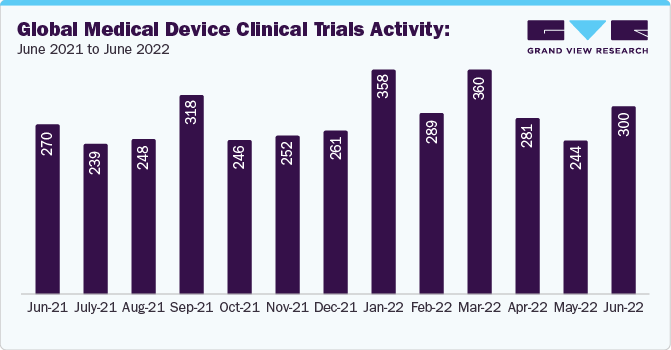
Case Study Insights: Octalsoft's CTMS Solution for a Pharmaceutical Company with Over 100 Clinical Trial Projects
Requirement:
-
Manage multiple global clinical trials
-
Comply with global regulatory requirements
-
Utilize CDISC-compliant electronic Trial Master File (eTMF) structure
-
Implement finance budget sheet based on countries
-
Streamline clinical operations by reducing manual tasks
Solution: Octalsoft CTMS, a centralized and customized solution, was chosen to capture data, monitor trial progress, generate reports, and address action items.
Outcome: The client achieved the following results:
-
Data-driven and real-time decision-making, leading to increased efficiency, cost reduction, adherence to timelines, and successful license filing
-
Increased productivity through task automation, real-time data sharing, and efficient time management
-
Flexible reporting capabilities with smart reports, dashboards, and various reporting tools tailored to specific needs
-
Improved document management and quality processes using Octalsoft eTMF
-
Establishment of an extensive database of verified principal investigators and clinical trial sites, providing a sustainable competitive advantage.
Market Concentration & Characteristics
Market players are investing in technological advancements, such as software upgrades, software and service integration, adoption of AI, and advanced analytics, to enhance their market presence and revenue. For example, in June 2023, Saama introduced an AI/ML-powered SaaS-based solution to automate clinical development processes and provide a comprehensive view of trial processes and patient progress in one platform.
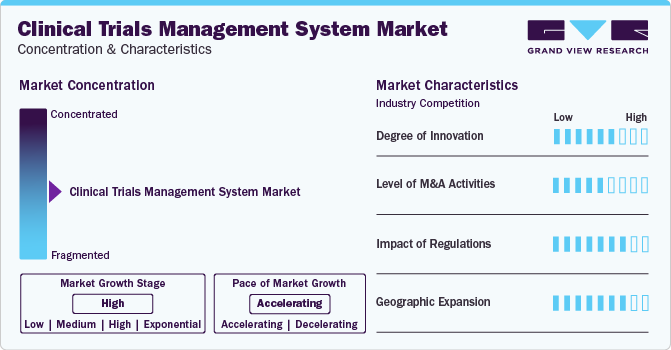
Many market players are acquiring smaller companies to bolster their position in the CTMS industry. This approach enables businesses to broaden their capabilities, diversify their product portfolios, and strengthen their expertise. For instance, in December 2022, RealTime Software Solutions, LLC acquired Complion, Inc., a move aimed at enhancing clinical study site management for Academic Medical Centers (AMCs), clinics, health systems, sponsors, and Contract Research Organizations (CROs).
CTMS industry players must adhere to various regulations to ensure the safety and security of clinical trials. Key guidelines include FDA 21 Code of Federal Regulations (CFR) Part 11, General Data Protection Regulation (GDPR), HIPAA, EU GMP Annex 11, and GCP compliance. Compliance with these regulations expedites the approval process for any software or system used in clinical trials.
CTMS companies employ geographic expansion strategies to sustain their presence in emerging markets and reach untapped regions, attracting customers from these areas. This strategy typically involves establishing new facilities or engaging in mergers and acquisitions in new locations.
Solution Type Insights
Based on solution type, the enterprise segment led the market with the largest revenue share of 74.35% in 2023. The market growth is attributed to key benefits, including providing comprehensive insights into deviations and accruals, robust reporting capabilities, scalable solutions, streamlined regulatory process management, and enhanced billing compliance. Enterprise software solutions are designed to integrate multiple facets or application packages, allowing organizations to handle various challenging tasks using a single software platform. For instance, Real Time's Enterprise CTMS offers comprehensive solutions for centralized recruitment, resource management, accounting, regulatory compliance, and aggregate reporting across universities and other large site networks.
The site segment is expected to grow at a significant CAGR during the forecast period, due to the associated benefits of onsite CTMS solutions. Site clinical trial management software solutions manage onsite contracts and payments, study subject rosters, calendar tracking, and document maintenance for small or medium-sized businesses. Some pharmaceutical companies prefer onsite software solutions to maintain complete control and access over the trial process. The use of local servers and VPN connections with onsite solutions further enhances data security, thereby contributing to the segment's growth.
Component Insights
Based on component, the software segment led the market with the largest revenue share of 56.04% in 2023. CTMS software is a user-friendly tool designed to streamline clinical trial management by overseeing regulatory procedures, trial planning, site and country progress, activity monitoring, finance, and supplies. It is commonly deployed at both site and enterprise levels, often through subscription-based models. In addition, CTMS software helps optimize and simplify clinical document management processes, ensuring data security and quality. Consequently, leading players in the industry are introducing products leveraging CTMS software to address various needs within healthcare organizations. For instance, in October 2023, Signant Health launched Signant Biotech, a clinical research methodology that utilizes software and services to meet the evolving requirements of small and medium-sized biopharmaceutical companies.
The services segment is anticipated to grow at the fastest CAGR during the forecast period. This growth is driven by increasing demand for training and assistance in software installation and system upgrades to keep pace with advancing IT technology. CTMS service providers offer cost-effective solutions to establish or enhance the technological infrastructure for efficient trial management. For example, PHARMASEAL's Engility, operating on a software-as-a-service model, offers an affordable enterprise CTMS suitable for organizations conducting multiple concurrent trials, ranging from small-scale to large-scale operations.
Delivery Mode Insights
Based on delivery mode, the web & cloud-based segment led the market with the largest revenue share of 70.82% in 2023. With widespread adoption among end-users, this segment is projected to maintain its dominance and exhibit rapid growth throughout the forecast period. These solutions facilitate clinical trial data management through third-party providers, offering scalable hosting options. In addition, cloud-based technologies offer the advantage of seamless data accessibility from various devices, including laptops, mobiles, workstations, and tablets, via CTMS software. Moreover, the introduction of cloud-based CTMS solutions by companies further fuels the market growth of this segment. For instance, in April 2021, Calyx launched Calyx CTMS v15.0, an enhanced CTMS on the Azure cloud platform, aimed at minimizing risks in clinical trial processes.
The on-premise segment is expected to grow at a significant CAGR over the forecast period. On-premise delivery involves installing solutions on computers or hardware within the organization. Despite requiring installation within the organization's premises, these solutions offer remote access, leading to reduced costs associated with power consumption and system maintenance. Furthermore, established companies often find it challenging to transition all their healthcare IT applications to the cloud. Consequently, some organizations opt for a hybrid approach, combining both on-premise and cloud-based services to create a flexible working environment.
End-user Insights
Based on end-user, the CRO and other segments held the market with the largest revenue share of 40.10% in 2023 and are anticipated to experience at the fastest CAGR throughout the forecast period. The market growth can be attributed to the rising number of partnerships, the outsourcing of clinical trials to contract research organizations, and the increasing prevalence of decentralized trials. For example, in September 2023, MedRhythms collaborated with Curavit Clinical Research, a CRO, for its decentralized clinical trial focusing on chronic stroke.
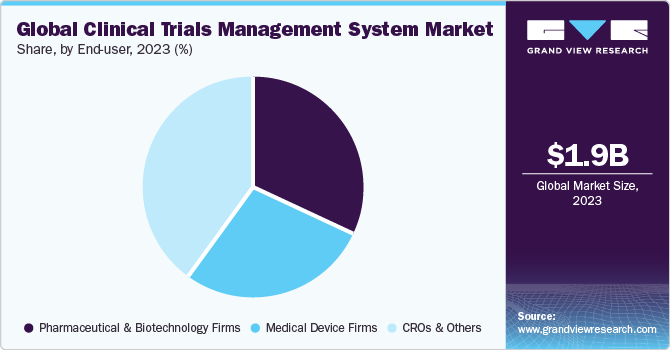
The medical device firms segment is expected to grow at a substantial CAGR during the forecast period. This growth is from the increased significance of enterprise CTMS within leading medical diagnostic and device organizations, aiming to streamline workflows, proactively address performance issues, and efficiently deploy critical resources. In addition, various governments have initiated efforts to promote the safe and effective conduct of clinical trials for medical devices. For instance, in May 2023, the U.S. FDA issued draft guidance for investigators, sponsors, and other stakeholders regarding the implementation of decentralized clinical trials (DCTs) for medical devices, drugs, and biological products. Consequently, the increasing number of clinical trials for medical devices is expected to drive the adoption of CTMS by medical device firms.
Regional Insights
North America dominated the clinical trials management system market with the revenue share of 50.01% in 2023. This dominance is driven by the presence of technologically advanced research institutions, universities, medical device manufacturers, and pharmaceutical companies, all of which actively utilize CTMS. Moreover, the region benefits from improved healthcare facilities. In addition, favorable government policies aimed at promoting clinical trials are anticipated to further boost the regional market. For instance, in January 2023, the Canadian government launched training platforms, a clinical trial consortium, and research projects aimed at enhancing the health outcomes of its citizens. Furthermore, approximately 22 projects received approximately USD 60.0 million in funding to support clinical trial designs, phases, and objectives aligned with the priorities of the Biomanufacturing and Life Sciences Strategy (BLSS).
U.S Clinical Trials Management System Market Trends
The clinical trials management system market in U.S. dominated the market in North America with the largest share of over 80.0% in 2023. Technological advancements in CTMS solutions have notably enhanced their accessibility and user-friendliness. In addition, numerous companies are strategically entering the national market to bolster their market standing. For example, in February 2024, Ergomed Group, a UK-based CTMS provider, extended its footprint in the U.S. by inaugurating a new office in Cambridge, Massachusetts.
Europe Clinical Trials Management System Market Trends
The clinical trials management system market in Europe is expected to grow at a substantial CAGR throughout the forecast period. This market growth is driven by the benefits associated with CTMS, including improved transparency, enhanced data quality and accuracy, and shortened timelines for clinical studies. Furthermore, organizations across Europe are implementing various initiatives to encourage clinical trials, thereby fostering increased adoption of CTMS. For example, in May 2023, the European Clinical Research Infrastructure Network (ECRIN) commemorated International Clinical Trial Day, with a particular emphasis on the challenges and opportunities presented by Decentralized Clinical Trials
The Germany clinical trials management system market is expected to grow at a significant CAGR during the forecast period. According to data from Verdict Media Limited, Germany contributed to 5% of global medical devices clinical trial activities in 2022. Moreover, the German Clinical Trials Register (DRKS) serves as the primary repository for conducted trial studies in the country, hosting over 15,000 trial studies, with an addition of 2,000 studies annually.
The clinical trials management system market in UK is also expected to witness at a substantial CAGR during the forecast period. The market growth is driven by initiatives spearheaded by the UK government to encourage clinical trials. For example, in May 2023, the government allocated USD 132.21 million in funding to accelerate clinical trials. This funding facilitated access to advanced technologies utilized in clinical trials, including CTMS solutions.
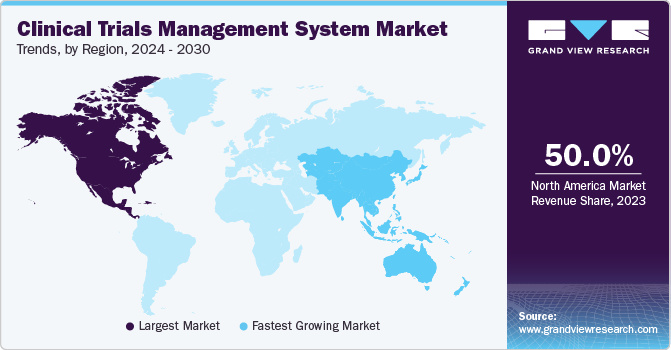
Asia Pacific Clinical Trials Management System Market Trends
The clinical trials management system market in Asia Pacific region is expected to grow at the fastest CAGR during the forecast period. This growth is driven by factors such as a substantial patient pool, increasing outsourcing trends, and significant investments by multinational corporations (MNCs) and contract research organizations (CROs) in clinical research and drug discovery. Key markets for clinical research outsourcing among major pharmaceutical and biotech companies include India and China, which are expected to drive the demand for CTMS solutions significantly.
The China clinical trials management system market is projected to witness at the significant CAGR during the forecast period. The country's high healthcare expenditure presents ample investment opportunities for clinical research expansion. Many companies in China are forming strategic partnerships to access CTMS solutions. For example, in December 2023, Oncoshot collaborated with Zhejiang Ablaze Medicine, a China-based provider of clinical trial management solutions, to develop an AI-driven drug development program.
The clinical trials management system market in Japan dominated the Asia Pacific in 2023. This growth is primarily attributed to the high volume of clinical trials conducted in the country. According to data from Verdict Media Limited, Japan accounted for 4.7% of global clinical trial activity in 2022, with 54.0% being industry-sponsored trials and 46.0% non-industry-sponsored.
Key Clinical Trials Management System Company Insights
The companies in the global market are actively involved in various strategic initiatives to sustain and grow.
Key Clinical Trials Management System Companies:
The following are the leading companies in the clinical trials management system market. These companies collectively hold the largest market share and dictate industry trends.
- IQVIA, Inc.
- Medidata (Dassault Systèmes)
- Oracle
- DATATRAK International, Inc.
- Clario
- SimpleTrials
- Calyx (formerly Parexel Informatics)
- RealTime Software Solutions, LLC
- Laboratory Corporation of America Holdings
- Veeva Systems
- Wipro Limited
- PHARMASEAL International Ltd.
Recent Developments
-
In January 2024, BSI Life Sciences announced its latest client, Ocular Therapeutix, for its cloud-based Clinical Trial Management System
-
In April 2022, Bristol Myers Squibb globally implemented Veeva Systems CTMS to drive end-to-end trial management. The implementation enabled Bristol Myers Squibb to establish agile, unified, and simple trial processes to make clinical trials swift and more efficient
-
In November 2022, the largest Korean CRO-C&R Research-expanded its collaboration with Medidata to improve clinical operations. This collaboration will strengthen C&R Research's ability to adapt to the quick clinical research environment to track clinical operations and manage data comprehensively
-
In October 2022, RealTime Software Solutions, LLC collaborated with Aspen Insights to integrate world-class EMR/EHR patient identification software into its world-class CTMS
-
In October 2022, Realtime Software Solutions launched the Beta phase of its ENGAGE! Family of software solutions, which includes MyStudyManager, the very first clinical trials site-based participant portal, and RealTime eCONSENT
Clinical Trials Management System Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 2.08 billion
Revenue forecast in 2030
USD 4.73 billion
Growth rate
CAGR of 14.64% from 2024 to 2030
Base year for estimation
2022
Historical data
2018 - 2023
Forecast period
2024 - 2030
Report updated
May 2024
Quantitative units
Revenue in USD million/billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Solution type, component, delivery mode, end-user, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
IQVIA, Inc.; Medidata (Dassault Systèmes); Oracle; DATATRAK International, Inc.; Clario; SimpleTrials; Calyx (formerly Parexel Informatics); RealTime Software Solutions, LLC; Laboratory Corporation of America Holdings; Veeva Systems; Wipro; PHARMASEAL International Ltd.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Clinical Trials Management System Market Segmentation
This report forecasts revenue growth and provides at global, regional, and country levels an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the global clinical trials management system market report based on solution type, component, delivery mode, end-user, and region:
-
Solution Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Enterprise
-
Site
-
-
Component Outlook (Revenue, USD Million, 2018 - 2030)
-
Software
-
Services
-
-
Delivery Mode Outlook (Revenue, USD Million, 2018 - 2030)
-
Web & Cloud Based
-
On Premise
-
-
End-user Outlook (Revenue, USD Million, 2018 - 2030)
-
Pharmaceutical and Biotechnology Firms
-
Medical Device Firms
-
CROs & Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global clinical trials management system market size was estimated at USD 1.85 billion in 2023 and is expected to reach USD 2.08 billion in 2024.
b. The global clinical trials management system market is expected to grow at a compound annual growth rate of 14.14% from 2024 to 2030 to reach USD 4.73 billion by 2030.
b. North America held approximately 50% of the clinical trials management system market in 2023. The large share of the North American region can be attributed to the presence of key companies and the rising adoption of technology in R&D.
b. Some key players operating in the CTMS market include IQVIA, Inc.; Medidata (Dassault Systèmes); Oracle; DATATRAK International, Inc.; Clario; SimpleTrials; Calyx (formerly Parexel Informatics); RealTime Software Solutions, LLC; Laboratory Corporation of America Holdings; Veeva Systems; Wipro; PHARMASEAL International Ltd.
b. Key factors that are driving the CTMS market growth include rapid growth of healthcare IT, preference for decentralized clinical trials, initiatives by key companies, and increasing number of clinical studies.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










