Market Size & Trends
The global non-invasive brain trauma monitoring devices market size was valued at USD 14.0 billion in 2023 and is projected to grow at a CAGR of 8.6% from 2024 to 2030. The rising incidences of Traumatic Brain Injuries (TBIs), increase in the awareness about brain disorders and increased use of brain monitoring in medical procedures are expected to positively impact the market growth. Furthermore, the market growth can be attributed to the increased funding in infrastructure, and rising need for non-invasive brain trauma monitoring devices-based solutions.
The market is growing due to the development in healthcare market with increase in investments for research and development in the field of brain disorders. Non-invasive brain trauma monitoring devices are used in minimally invasive neurosurgical treatment. With rise in the traumatic brain injuries, patients prefer minimally invasive procedures, as they are less traumatic and more effective allowing the patient to recover faster. The non-invasive brain trauma monitoring allows medical professional continuously monitor the brain helping them to take appropriate action in order to reduce the brain trauma. Furthermore, with growth in the geriatric population, the number of TBIs is also increasing, leading to market growth due to aging being a significant factor in the development of neurological disorders.
Product Insights
Monitoring devices segment dominated the market and accounted market share of 63.2% in 2023 majorly due to the rise in the preference of using non-invasive procedures for the treatment of brain disorders. The monitoring devices is further segmented into intra cranial pressure monitors, MRI scanner, CT scanner, EEG, MEG, and other devices. Cost of these devices is much higher than the consumables therefore helping in revenue generation. The market is further anticipated to grow in the healthcare sector as medical institutes aim to be equipped with latest monitoring devices for treatments.

Consumables segment is expected to witness the fastest CAGR of 9.2% over the forecast period. Consumables segment is further divided into electrodes, sensors, fiber optic cables, and others. The major reason for the market growth of this segment is the increased prevalence of brain disorders, which has increased the demand in production of this segment. Frequent replacements of these items during maintenance has further attributed to the market growth.
End-use Insights
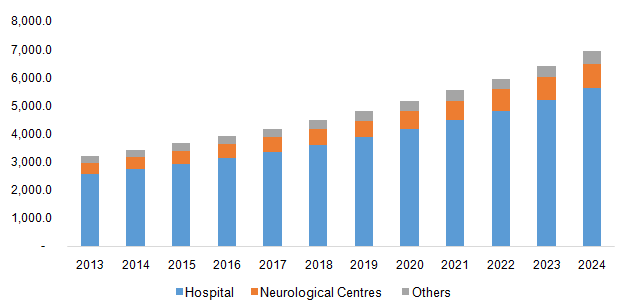
The hospitals segment dominated the market and accounted for a share of 80.9% in 2023 attributing to the significant rise in the brain surgeries conducted in hospitals worldwide. Furthermore, the preference of hospitals over other facilities has increased due to the technological advancements in the devices used for treatments in the hospitals. Hospitals are also economically capable of handling the procurement and maintenance of these devices therefore generating revenue for this market.
The neurological centres segment is anticipated to grow at the fastest CAGR of 9.3% over the forecast period. The availability of specialized medical professionals who perform treatments efficiently has resulted in increase in the preference of this segment. The market growth is also supported by the rise in the disposable income of population in developing economies that choose non-invasive treatments in order to treat brain disorders.
Regional Insights
North America dominated the non-invasive brain trauma monitoring devices market with a share of 42.6% in the year 2023. It is attributable to increased adoption of non-invasive brain trauma monitoring devices and the presence of major medical institutes with their heavy investments in the technological advance of medical devices. The increase in geriatric population suffering from brain disorders has further resulted in the market growth of this region.

U.S. Non-invasive Brain Trauma Monitoring Devices Market Trends
The non-invasive brain trauma monitoring devices market in the U.S. accounted for a market share of 75.3% in 2023 due to the rise in disposable income of population and presence of major medical institutes in the country. Increase in investment of technological advancements in medical sector by government and medical institutes has further helped in improving the market growth in the country.
Europe Non-invasive Brain Trauma Monitoring Devices Market Trends
Europe non-invasive brain trauma monitoring devices market was identified as a lucrative region in this industry with a market share of 30.3% in 2023 due to the increase in patients suffering from neurological brain disorders. Furthermore, substantial investments by companies in non-invasive brain trauma monitoring devices and rise in preference of non-invasive treatments has contributed to the growth this region.
The UK non-invasive brain trauma monitoring devices market is expected to grow rapidly in the coming years due to the rise in medical institutes using non-invasive monitoring devices to treat brain disorders.
Asia Pacific Non-invasive Brain Trauma Monitoring Devices Market Trends
Asia Pacific non-invasive brain trauma monitoring devices market had a market share of 19.8% attributed to the technological growth in healthcare segment and increase in the patients of neurological brain disorders. The market is poised to grow with the improvement in economic condition of countries such as Japan and India.
China non-invasive brain trauma monitoring devices market held a substantial market share in 2023 owing to the rapid urbanization and presence of strong manufacturing facilities. Furthermore, the market growth is attributed to the initiative taken by government to improve medical facilities.
Key Non-invasive Brain Trauma Monitoring Devices Company Insights
Some of the major companies in the non-invasive brain trauma monitoring devices market are Advanced Brain Monitoring, Inc., Cadwell Industries, Inc., Canon Medical, Systems Corporation, Compumedics Ltd. Companies in this market are focusing on implementing new strategies such as incorporating new technology, developing new products, making mergers & acquisitions, forming joint ventures, alliances, and partnerships to enhance their market position in the global non-invasive brain trauma monitoring devices industry.
-
Advanced Brain Monitoring, Inc. is a neuro-diagnostics device company offering services such as sleep profiler diagnostics, night shift therapy, STAT X-series neurotechnology, apnea guard therapy and more.
-
Cadwell Industries, Inc. is a provider of neurodiagnostic, neuromonitoring, and sleep diagnostic solutions. They provide services such as sleep diagnosis, neuro consumables, Intraoperative Neuromonitoring (IONM) and more.
Key Non-invasive Brain Trauma Monitoring Devices Companies:
The following are the leading companies in the non-invasive brain trauma monitoring devices market. These companies collectively hold the largest market share and dictate industry trends.
- Advanced Brain Monitoring, Inc.
- Cadwell Industries, Inc.
- Canon Medical Systems Corporation
- Compumedics Ltd.
- Magstim EGI
- General Electric Company
- Hitachi, Ltd.
- Koninklijke Philips N.V.
- Medtronic
- Natus Medical Incorporated
- NeuroLogica Corp.
- NeuroWave Systems, Inc.
- Nihon Kohden Corporation
- Noraxon USA
- RAUMEDIC AG
- Sense Neuro
- Siemens
- Sophysa
- Spiegelberg GmbH & Co. KG
Recent Developments
-
In May 2024, Canon Medical Systems launched the first installation of the Aquilion Serve SP CT scanner in the U.S. INSTINX is an AI-powered workflow automation system. This scanner is expected to blend advanced imaging features with improved efficiency, consistency, and throughput.
Non-invasive Brain Trauma Monitoring Devices Market Report Scope
|
Report Attribute
|
Details
|
|
Market size value in 2024
|
USD 15.1 billion
|
|
Revenue forecast in 2030
|
USD 24.8 billion
|
|
Growth Rate
|
CAGR of 8.6% from 2024 to 2030
|
|
Base year for estimation
|
2023
|
|
Historical data
|
2018 - 2022
|
|
Forecast period
|
2024 - 2030
|
|
Report updated
|
September 2024
|
|
Quantitative units
|
Revenue in USD billion and CAGR from 2024 to 2030
|
|
Report coverage
|
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
|
|
Segments covered
|
Product, end-use, region
|
|
Regional scope
|
North America; Europe; Asia Pacific; Latin America; MEA
|
|
Country scope
|
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
|
|
Key companies profiled
|
Advanced Brain Monitoring, Inc.; Cadwell Industries, Inc.; Canon Medical Systems Corporation; Compumedics Ltd.; Magstim EGI; General Electric Company; Hitachi, Ltd.; Koninklijke Philips N.V.; Medtronic; Natus Medical Incorporated; NeuroLogica Corp.; NeuroWave Systems, Inc.; Nihon Kohden Corporation; Noraxon USA; RAUMEDIC AG; Sense Neuro ; Siemens; Sophysa; Spiegelberg GmbH & Co. KG
|
|
Customization scope
|
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
|
|
Pricing and purchase options
|
Avail customized purchase options to meet your exact research needs. Explore purchase options
|
Global Non-invasive Brain Trauma Monitoring Devices Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global non-invasive brain trauma monitoring devices market report based on product, end-use, and region:

-
Product Outlook (Revenue, USD Billion, 2018 - 2030)
-
Monitoring Devices
-
Consumables
-
Electrodes
-
Sensors
-
Fibre Optic Cables
-
Others
-
End-use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Hospitals
-
Neurological Centres
-
Others
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)















