- Home
- »
- Healthcare IT
- »
-
Post-marketing Pharmacovigilance & Medical Information Market 2030GVR Report cover
![Post-marketing Pharmacovigilance And Medical Information Market Size, Share & Trends Report]()
Post-marketing Pharmacovigilance And Medical Information Market (2024 - 2030) Size, Share & Trends Analysis Report By Service (Spontaneous Reporting, Intensified ADR Reporting), By End Use, By Region, And Segment Forecasts
- Report ID: 978-1-68038-758-2
- Number of Report Pages: 100
- Format: PDF
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Market Size & Trends
The global post-marketing pharmacovigilance and medical information market size was valued at USD 5.60 trillion in 2023 and is projected to grow at a CAGR of 9.5% from 2024 to 2030. Post-market surveillance, encompassing pharmacovigilance and medical information provision, is crucial for ensuring product safety, quality, and efficacy. The growing demand for pharmaceuticals stems from the increasing elderly population and rising prevalence of chronic illnesses, such as diabetes, memory loss, heart disease, and osteoporosis, worldwide.
The growing global geriatric population and rising prevalence of chronic diseases, such as diabetes, hypertension, dementia, cardiovascular diseases, and osteoporosis, are leading to increased demand for medications. This, in turn, is driving market growth as the elderly population requires more frequent medication and treatment. Moreover, government initiatives to improve healthcare infrastructure, pharmacovigilance services, and medical databases are also strengthening market growth.
The expansion of healthcare infrastructure, including the increasing number of hospitals, clinics, laboratories, and medical research centers, is catalyzing the demand for medications and medical devices globally. Another key driver of the market is the rising incidence of Adverse Drug Reactions (ADRs) and the incorporation of advanced ADR reporting tools. The growing number of ADRs, coupled with the adoption of improved ADR reporting technologies, is supporting market expansion.
The market is also being driven by the outsourcing of pharmacovigilance operations and the introduction of cloud-based medical information systems. Leading players are focusing on outsourcing pharmacovigilance activities and introducing cloud-based systems for improved accuracy, cost efficiency, risk mitigation, and organizational agility. This shift towards outsourcing is revolutionizing the post-marketing pharmacovigilance market. Furthermore, the growing awareness among pharmaceutical staff about the importance of reporting adverse drug reactions and participating in pharmacovigilance work is also contributing to improved reporting speed and data quality in pharmacovigilance databases.
Service Insights
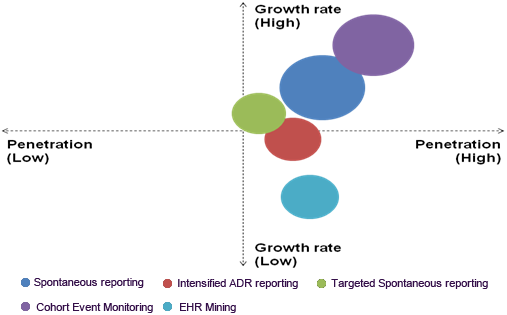
The spontaneous reporting service segment dominated the post-marketing pharmacovigilance and medical information market with a revenue share of 35.0% in 2023. Real-time data collection, early adverse event detection, and wide accessibility enable pharmacovigilance systems to dominate the market. Compliance with regulatory requirements, signal detection, and risk management capabilities contribute to its success. Spontaneous reporting services facilitate rapid identification of adverse events, allowing for timely detection of new risks and ensuring drug safety.
Thecohort event monitoring service segment is expected to register the fastest CAGR of 16.1% over the forecast period. Cohort event monitoring provides a proactive approach to tracking unfavorable activities in patients receiving specific medications or vaccines. This methodology involves enrolling patient cohorts, collecting real-world data through observational studies and post-marketing surveillance, and analyzing longitudinal trends to inform treatment decisions. Insights gained from this approach enable healthcare professionals to make more informed choices.
End-use Insights
The hospital segment dominated the post-marketing pharmacovigilance and medical information market with a revenue share of 59.1% in 2023. The industry’s dominance is driven by the need for enhanced patient safety, regulatory compliance, and the growing demand for evidence-based medicine. Hospitals prioritize patient safety by investing in robust post-marketing pharmacovigilance and medical information systems, enabling healthcare professionals to identify, monitor, and assess potential drug side effects, ultimately ensuring high-quality care with minimal risks.
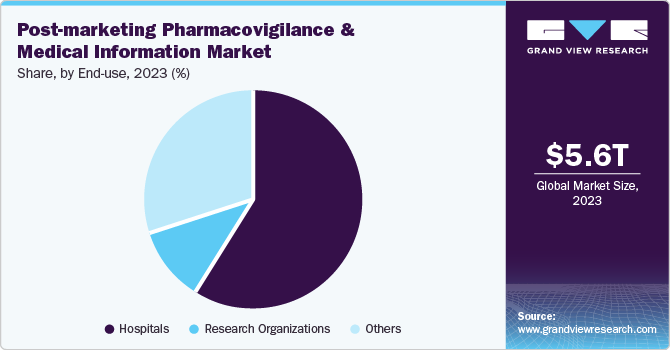
The research organizations segment is expected to register the fastest CAGR of 11.3% over the forecast period. The growth can be attributed to the organization’s expertise, global reach, regulatory partnerships, innovative thinking, and adaptability, enabling them to drive change. Leveraging advanced technology and connections to physicians, they efficiently monitor drug safety, collecting, analyzing, and disseminating data. This enables research teams to manage vast amounts of information while ensuring compliance with regulatory requirements.
Regional Insights
North America post-marketing pharmacovigilance and medical information market dominated the global post-marketing pharmacovigilance and medical information market with a revenue share of 39.1% in 2023. The region’s dominance can be attributed to its strong regulatory framework, cutting-edge technology, substantial market size, and highly skilled workforce. In addition, the well-established healthcare system and expert professionals in the region are driving growth further, as they provide high-quality services and foster innovation. This combination of factors enables the organization to maintain its competitive edge.
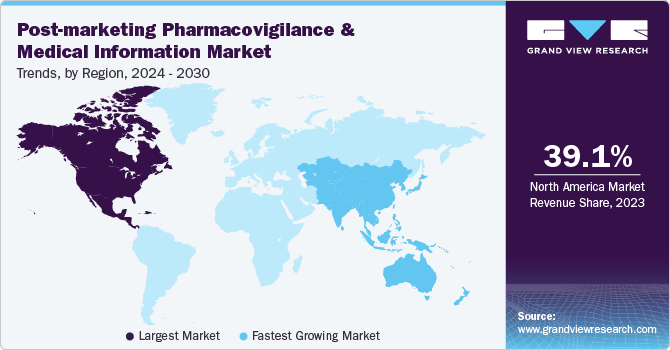
U.S. Post-marketing Pharmacovigilance And Medical Information Market Trends
The post-marketing pharmacovigilance and medical information market in the U.S. dominated the North America post-marketing pharmacovigilance and medical information market with a revenue share of 64.0% in 2023. The U.S. is at the forefront of technological innovation, leveraging advanced data analytics, AI, and system mastering skills to drive drug safety monitoring. This tech advantage enables American companies to deliver premium solutions for tracking adverse reactions and analyzing real-world data, setting them apart in the global market.
Europe Post-marketing Pharmacovigilance And Medical Information Market Trends
Europe post-marketing pharmacovigilance and medical information market was identified as a substantial region in the global in 2023. Europe has a strong regulatory framework for pharmacovigilance, with organizations such as the European Medicines Agency (EMA) playing a key role in monitoring the protection of drugs put up and approved. The stringent guidelines and reporting requirements in Europe have contributed to constructing a culture of pharmacovigilance excellence within the region.
The post-marketing pharmacovigilance and medical information market in Germany is expected to grow over the forecast period. Germany has a top-notch healthcare system with cutting-edge facilities, state-of-the-art tech, and skilled medical staff. This setup has an impact on making pharmacovigilance work and allows for quick gathering, study, and sharing of drug safety data.
The UK post-marketing pharmacovigilance and medical information market held a substantial market share in 2023. The UK has a robust regulatory framework for pharmacovigilance, with agencies such as the Medicines and Healthcare Products Regulatory Agency (MHRA) overseeing drug protection monitoring and reporting. The stringent regulations make sure that pharmaceutical agencies running in the UK adhere to excessive standards of pharmacovigilance practices, thereby enhancing the country’s reputation in the field.
Asia Pacific Post-marketing Pharmacovigilance And Medical Information Market Trends
The post-marketing pharmacovigilance and medical information market in Asia Pacific is expected to register the fastest CAGR of 11.3% over the forecast period. One of the number one reasons for Asia Pacific’s lucrative growth in post-advertising pharmacovigilance and medical information is the region’s rapidly growing pharmaceutical enterprise. The Asia Pacific region is home to a massive and diverse population base, which offers a vast marketplace for pharmaceutical corporations. Moreover, Asia Pacific gives cost-effective solutions for the post-marketing pharmacovigilance and medical information market.
China post-marketing pharmacovigilance and medical information market is expected to grow over the forecast period. China has applied stringent rules and recommendations to ensure the safety of pharmaceutical products in the post-marketing phase. With a massive population and a growing healthcare sector, China represents a rewarding market for post-marketing pharmacovigilance offerings.
The post-marketing pharmacovigilance and medical information market in India held a substantial market share in 2023. India has emerged as a hub for pharmacovigilance and medical information services due to its cost-effective solutions. The availability of skilled specialists at competitive rates has attracted pharmaceutical companies to outsource their pharmacovigilance activities to Indian firms.
Key Post-marketing Pharmacovigilance And Medical Information Company Insights
Some key companies in the post-marketing pharmacovigilance and medical information market include IQVIA Inc., Parexel International (MA) Corporation, Laboratory Corporation of America Holdings., ICON plc, Syneos Health., Organizations are focusing on increasing customer base to gain a competitive edge in the industry. The key players are taking several strategic initiatives, such as mergers and acquisitions, and partnerships with other major companies.
-
IQVIA Inc., a provider of superior analytics, technological solutions, and medical research services to the life sciences industry, focuses on developing intelligent connections across all elements of healthcare. The company focuses on the scientific improvement and commercialization of modern scientific remedies that enhance healthcare outcomes for patients.
-
Syneos Health is a biopharmaceutical solution organization that specializes in translating clinical, clinical affairs, and commercial insights into results that address modern-day marketplace realities.
Key Post-marketing Pharmacovigilance And Medical Information Companies:
The following are the leading companies in the post-marketing pharmacovigilance and medical information market. These companies collectively hold the largest market share and dictate industry trends.
- IQVIA Inc
- Parexel International (MA) Corporation
- Laboratory Corporation of America Holdings
- ICON plc
- Syneos Health
- Accenture
- Cognizant
- F. Hoffmann-La Roche Ltd
- Sanofi
- ArisGlobal
- Ergomed Group
- Medpace
- IBM Corporation
- Wipro
- Capgemini
Recent Developments
-
In June 2024, IQVIA introduced a home clinical technology trial platform, addressing site challenges and reducing workload. This technology enabled sites to streamline recruitment and treatment processes, increasing patient enrollment capacity and concurrent trial management.
-
In February 2023, Labcorp announced the formation of Fortrea, a new publicly traded CRO, resulting from the deliberate spin-off of its clinical development business. Fortrea now operates as an independent company, offering comprehensive drug and device development services globally.
Post-marketing Pharmacovigilance And Medical Information Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 6.10 trillion
Revenue forecast in 2030
USD 10.50 trillion
Growth rate
CAGR of 9.5% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Service, end use, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, Mexico, UK, Germany, France, Italy, Spain, Denmark, Sweden, Norway, China, Japan, India, South Korea, Australia, Thailand, Brazil, Argentina, Saudi Arabia, Kuwait, UAE, South Africa
Key companies profiled
IQVIA Inc; Parexel International (MA) Corporation; Laboratory Corporation of America Holdings; ICON plc; Syneos Health; Accenture; Cognizant; F. Hoffmann-La Roche Ltd; Sanofi; ArisGlobal; Ergomed Group; Medpace; IBM Corporation; Wipro; Capgemini
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Post-marketing Pharmacovigilance And Medical Information Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global post-marketing pharmacovigilance and medical information market report based on service, end use, and region.
-
Service Outlook (Revenue, USD Billion, 2018 - 2030)
-
Spontaneous reporting
-
Intensified ADR reporting
-
Targeted spontaneous reporting
-
Cohort event monitoring
-
EHR mining
-
-
End Use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Hospitals
-
Research Organizations
-
Others
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
MEA
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










