- Home
- »
- Market Trend Reports
- »
-
Viral Vector Development - Focus on Adeno-associated Virus
![Viral Vector Development - Focus on Adeno-associated Virus, 2018 - 2021Report]()
Viral Vector Development - Focus on Adeno-associated Virus, 2018 - 2021
- Published: Apr, 2023
- Report ID: GVR-MT-100108
- Format: PDF, Horizon Databook
- No. of Pages/Datapoints: 30
- Report Coverage: 2024 - 2030
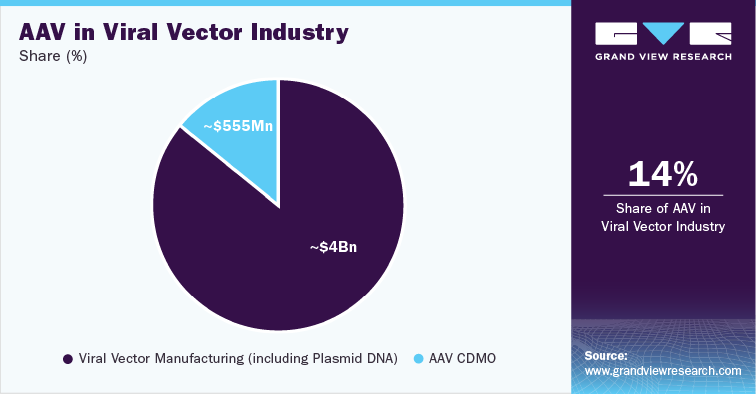
Adeno-associated Viruses (AAVs) in Gene Therapy Development
Gene therapy is a promising field in the medical industry, with the potential to revolutionize current treatment options. One of the main platforms for delivering gene therapies is using Adeno-associated Viruses (AAVs). AAVs are nonpathogenic, they do not cause disease-and they are highly capable of effectively delivering genetic material to cells. As a result, they have become a popular choice in gene therapy development. In addition, advancements in manufacturing technology for AAV vectors and the various strategies being implemented by market players are also driving the growth of the AAV CDMO market.
Viral Vector Report Scope
Attribute
Details
Market Lineage
- % Captured by AAV within Viral Vectors
- Understanding of the parent market and ancillary industries
- Plasmid DNA Contract Manufacturing
- Cell & Gene Therapy Clinical Trials Market
Industry Dynamics
- Viral Vector based gene therapy development
- AAV Development workflow
- Pipeline for AAV
- Capitalist funding in the industry
- Development Process
Deep dive Analysis
- Understanding of AAV v/s Lentiviral v/s other viral vectors
- Cost of manufacturing (based on batch size) - a case study
- Case study on setting up a development plant
Competition Analysis in CDMOs
- Companies with products in clinical trials
- Various bioreactors used for development
- Strategic initiatives undertaken in the last 5 years
Observation and Recommendations
This section will summarize the entire study and provide analyst insights with regards to key methods of development, technological advancements, and competitive profiling
Viral vectors are tools for delivering genetic material into cells. Viruses have developed specific systems for transporting their DNA inside the cells they infect. Furthermore, retrovirus, adenovirus, lentivirus, herpes simplex virus, and others are among the viral vectors which can be employed to transfer genetic material into the genetic composition of cells. The market for viral vectors and plasmid DNA manufacturing is growing due to the increasing prevalence of target ailments and diseases and the efficacy of viral vectors in gene therapy delivery.
This increase is aided by continued research into viral vector-based cell and gene therapies, as well as financing for gene therapy advancement. In addition, an increase in the number of gene therapy-based discovery programs initiated by biotechnology and pharmaceutical companies is expected to drive the demand for scalable production of gene therapy vectors. Also, the ongoing COVID-19 pandemic has encouraged investment in this space for vaccine development against SARS-CoV-2, thereby driving the market for viral vector production (research-use)
In 2020, there were more than 100 gene-therapy products in clinical trials, with a large number still in preclinical research. The potential of viral vectors has gained the attention of large pharma companies, with the acquisition of seven biotech startups in the last 2 years, valued at around USD 1 billion. With the approval of adenovirus-based COVID-19 & Ebola vaccines in the past year, adenoviruses have demonstrated their efficacy as a vaccine platform.
AAV vectors were the first in vivo gene therapy products to be approved for use in Western markets. These include FDA-approved drugs Luxturna (voretigene neparvovec, 2017) & Zolgensma (onasemnogene abeparvovec-xioi, 2019), and Glybera (alipogene tiparvovec), which was also approved by the European Medicines Agency (EMA) in 2012. Furthermore, rAAV vectors were involved in almost all the therapeutic viral vectors, which EMA designated as priority medicines in 2017.
Therapies using AAVs vector type
Drug Name
Company
Indication
Approval Date
Luxturna
Spark Therapeutics
Retinal dystrophy
2017, FDA
2018, EMA
Zolgensma
AveXis
Spinal muscular atrophytype 1
2019, FDA
2020, EMA
Gene therapies have the potential to revolutionize the way diseases are addressed, by treating the underlying genetic disorder. However, there are multiple regulatory and
developmental difficulties to be addressed, given the intricacy of gene treatments as well as how rapidly the area is advancing. The manufacturing of AAV viral vectors is an intricate process and needs innovative approaches to meet efficacy & safety requirements, the total cost of virus production, and clinical & market demands.
Major challenges for AAV manufacturers include the preparation of stable viral vectors, preventing their degradation during manufacturing, storage & handling, and maintaining long-term efficacy & safety. The cost is another key aspect impacting development. AAVs used for research purposes may be priced differently than those used for clinical trials or commercial products. Additionally, the cost may vary depending on the supplier and the specific terms of the purchase. It is difficult to provide a specific cost without more information. According to an article published by Jecob Pelith on Evaluate Vantage, the cost of manufacturing a single dose of AAV with the titer of 8x10^15 comes to around USD 100,000. An in-depth analysis of the manufacturing cost shall be provided in the report.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


