- Home
- »
- Medical Devices
- »
-
Interventional Cardiology Devices Market Size Report, 2030GVR Report cover
![Interventional Cardiology Devices Market Size, Share & Trends Report]()
Interventional Cardiology Devices Market Size, Share & Trends Analysis Report By Product (Coronary Stents, PTCA Balloon Catheters, Accessory Devices), By Region, And Segment Forecasts, 2023 - 2030
- Report ID: 978-1-68038-122-1
- Number of Pages: 150
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Interventional Cardiology Devices Market Size & Trends
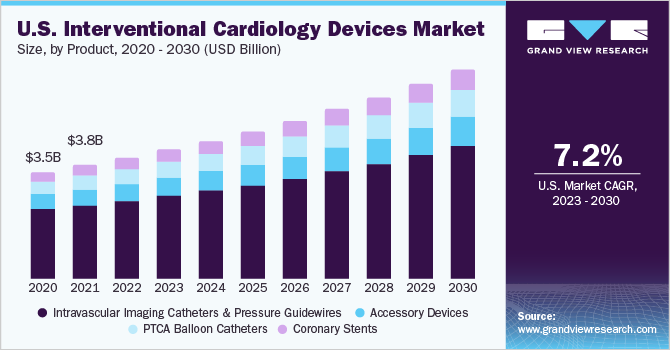
The global interventional cardiology devices market size was valued at USD 12.91 billion in 2022 and is anticipated to grow at a compound annual growth rate (CAGR) of 7.2% from 2023 to 2030. Technological advancements, the launch of new products, favorable government policies, and increasing incidence rates of heart valve disease, Carotid Artery Disease (CAD), and peripheral artery disease are expected to propel market growth. Moreover, the increasing penetration & awareness about Minimally Invasive Surgeries (MIS) among the global population is further expected to drive market growth. According to the National Center for Biotechnology Information (NCBI), over ten units are currently undertaking cardiac MIS in the UK National Health Service (NHS).
Government initiatives to increase the accessibility and cost of IC treatments, such as the Ayushman Bharat Yojana in India, would encourage patient numbers to rise substantially during the forecast period. In September 2018, the Indian government introduced the largest publicly financed healthcare program in the world, the Ayushman Bharat Yojana. Focusing on disadvantaged families in rural areas, the government hopes to create a network of primary healthcare facilities centered in the community and offer insurance coverage for secondary and tertiary services to roughly half of the nation's residents.
Non-profit medical associations are also contributing to the growth of the market. For instance, in September 2023, the Society for Cardiovascular Angiography & Interventions (SCAI), a leading non-profit medical society, is coming up with the 6th Annual SCAI Interface in Interventional Cardiology in Mumbai, India, in the presence of leading interventional cardiologists from around the globe.
The COVID-19 pandemic had a significant impact on the interventional cardiology device market. The industry experienced a decline in demand as elective procedures were postponed or canceled to curb the spread of the virus. As per data from the British Cardiovascular Intervention Society, PCI volumes in the UK decreased by 49% during the lockdown phase of the pandemic. This decline was most pronounced in PCI for stable angina, which witnessed a 66% volume reduction. Other studies showed similar declines in interventional cardiology procedures in other nations. For instance, a study in the U.S. highlighted that PCI volumes decreased by 30% in the first few months of the pandemic.
Product Insights
The coronary stents segment dominated the market with the largest revenue share of 64.6% in 2022. DES makes up the majority of sales in the coronary stent category. The market has benefited from the introduction of next-generation DES such as the XIENCE series by Abbott Laboratories, Medtronic's Resolute Onyx, and many other well-known stents, including the SYNERGY and Orsiro DES from Boston Scientific and BIOTRONIK, respectively. These stents provide superior deliverability, stent integrity, and reduced rates of problems compared to their older counterparts.
In August 2022, Medtronic announced that it had received the European CE Mark approval for its Onyx Frontier Drug-Eluting Stent (DES). Also, Onyx Frontier had received U.S. FDA approval in May 2022. This DES offers significant improvements over the company’s previous generation Resolute Onyx DES family. It features an enhanced delivery system designed to improve deliverability and increase acute performance.
The market for PTCA balloon catheters will also continue to rise significantly throughout the forecast period, partly because more PCI procedures are expected to be performed, especially in places such as India, China, and Latin American nations, where untreated CAD is more prevalent. The total number of IC processes significantly influences the growth of the market for companion devices. As more patients in the covered regions become eligible for Percutaneous Coronary Intervention (PCI), accessory device sales are expected to increase, driving segment revenues.
As several devices must be used in every IC method, the accessory device market is rather large, despite typically low ASPs compared to other device categories. For instance, in August 2022, Kossel Medtech launched its first peripheral product, the Falspeed percutaneous transluminal angioplasty (PTA) balloon dilatation catheter, approved by the National Medical Products Administration (NMPA) in June 2022.
The company further announced that the PTA balloon catheter is also registered under the FDA and is expected to receive 510(K) clearance by the end of 2022. In another instance, in February 2022, CERENOVUS, a part of Johnson & Johnson Medical Devices Companies, launched EMBOGUARD. This next-generation balloon guide catheter has been indicated for use in patients with acute ischemic stroke and also in endovascular procedures.
The intravascular imaging catheters and pressure guidewires segment is expected to expand at the fastest CAGR of 8.5% over the forecast period. These devices are used in minimally invasive procedures, which are becoming increasingly popular due to their lower risk of complications, shorter recovery times, and advancements in intravascular imaging technologies, such as Intravascular Ultrasound (IVUS) and Optical Coherence Tomography (OCT). They offer more detailed images of the heart and coronary arteries, which can help physicians make more informed treatment decisions.
Several developments support the growth of this segment. For instance, Intravascular Imaging Incorporated (i3) collaborated with Massachusetts General Hospital (Boston) to design a 3-French NIRF-IVUS catheter. The product, launched in July 2022, helps visualize cellular and molecular details, which underlines the pathophysiologic progression of arterial dissections, coronary lesions, and coronary transplant vasculopathy.
The reimbursement scenario is also favorable for atherectomy, contributing to its growth. For instance, in January 2023, the French National Authority for Health (HAS) released recommendations regarding add-on reimbursement of medical devices and aids, which included opinions concerning peripheral vascular and cardiovascular devices. The reimbursement suggestions had been gathered from the meetings of the National Commission for Evaluation of Medical Devices and Health Technologies (CNEDiMTS).
Regional Insights
North America dominated the market with the largest revenue share of around 36.5% in 2022, owing to the high prevalence of cardiovascular diseases, such as coronary artery disease and heart failure, in the region. This is due to several factors, including the high prevalence of obesity, smoking, and diabetes among the regional population. Advanced healthcare infrastructure, including the establishment of several hospitals and clinics, and high disposable incomes are other factors propelling the regional market growth.
For instance, in April 2022, New York-Presbyterian Hudson Valley Hospital announced the launch of an Interventional Cardiology Program. Along with this, the facility also launched an advanced cardiac catheterization lab to provide advanced cardiac intervention services and an expanded access to leading specialists and care. Such initiatives are expected to drive the growth of the interventional cardiology devices market.
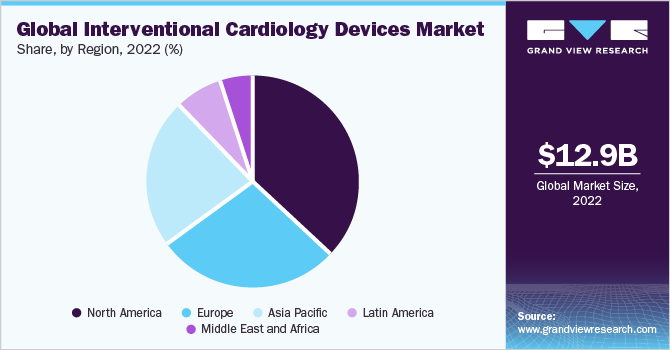
On the other hand, the Asia Pacific region is expected to advance at the fastest CAGR of 7.9% over the forecast period. This growth can be attributed to several factors, including the increasing incidence of coronary artery disease (CAD) and improved screening measures for this disorder. Furthermore, the region's economic development, augmented government investment in healthcare, favorable reimbursement policies in countries such as South Korea and Australia, and the expansion of healthcare infrastructure in India and China have contributed to this trend.
Additionally, the escalating prevalence of various CAD risk factors, including unhealthy diets, smoking, and sedentary lifestyles, has played a vital role in driving regional growth. The Chinese government implemented a centralized procurement procedure for high-value medical consumables in January 2021, significantly reducing the costs of DES. In November 2021, Medtronic launched the Arctic Front Cardiac Cryoablation Catheter System in India. It became the first and only cryoballoon catheter for treating atrial fibrillation (AF) approved by the Central Drugs Standard Control Organization (CDSCO).
In another development, in February 2023, Swiss Interventional Systems (SIS) Medical AG launched the OPN NC percutaneous transluminal coronary angioplasty (PTCA) catheter in the U.S. It is a dilatation catheter with Twin-Wall technology that was approved by the FDA in March 2022. Also, in September 2022, Lepu Medical Technology Co., Ltd. announced that it had recieved approval from the NMPA to launch its peripheral cutting balloon for treating PAD (peripheral arterial disease) in Beijing, China.
Europe is anticipated to witness exponential growth during the forecast period due to ongoing innovations, regional approval of newer technologies such as next-generation DES and BRS, and subsequent acceptance of these expensive technologies due to their benefits in revascularization treatments. For instance, in May 2023, the European Society of Cardiology collaborated with Europa Group and PCR to reduce the global spread of cardiovascular disease. In May 2023, Canon Medical joined an advanced course in interventional cardiovascular medicine at the EuroPCR conference in France, to learn about the latest dose-reduction devices & tools and cutting-edge technologies.
Key Companies & Market Share Insights
The market for interventional cardiology devices is highly competitive and companies are undertaking strategies such as new product launches and technological advancements to increase their market penetration. For instance, in November 2022, Medical Manufacturing Technologies (MMT), a provider of medical device manufacturing solutions, announced the acquisition of the equipment manufacturing group of Confluent Medical Technologies, thus bringing in the manufacturing capabilities of balloon forming equipment and testing & catheter manufacturing equipment.
Similarly, in August 2021, Biomerics LLC, a manufacturer of interventional medical devices, announced the acquisition of a majority stake in Berg Manufacturing Inc., a company operating in the robotic surgery, endoscope, and, interventional catheters space. Following are some of the major participants in the global interventional cardiology devices market:
-
Abbott Laboratories
-
Medtronic
-
Boston Scientific
-
Spectranetics
-
Cardinal Health
-
Philips Healthcare
-
Terumo
-
Alvimedica
-
Teleflex Medical
-
B. Braun
-
Biosensors International
-
Meril Life Sciences
-
BIOTRONIK
-
ACIST Medical Systems
-
ASAHI INTECC
-
CID Vascular
-
Cook Medical
-
Medinol
-
Merit Medical Systems
-
NuMED
-
OpSens
-
Vascular Solutions
-
Zeon Medical
Interventional Cardiology Devices Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 13.75 billion
Revenue forecast in 2030
USD 22.35 billion
Growth Rate
CAGR of 7.2% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Key companies profiled
Abbott Laboratories; Medtronic; Boston Scientific; Spectranetics; Cardinal Health; Philips Healthcare; Terumo; Alvimedica; Teleflex Medical; B. Braun; Biosensors International; Meril Life Sciences; BIOTRONIK; ACIST Medical Systems; ASAHI INTECC; CID Vascular; Cook Medical; Medinol; Merit Medical Systems; NuMED; OpSens; Vascular Solutions; Zeon Medical
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Interventional Cardiology Devices Market Report Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global interventional cardiology devicesmarket report based on product and region:

-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Coronary Stents
-
DES
-
BMS
-
BRS
-
-
PTCA Balloon Catheters
-
Normal
-
Specialty (Includes cutting, scoring, and lithotripsy balloons)
-
DCB
-
-
Accessory Devices
-
PTCA Guidewires (Workhorse, Specialty)
-
Diagnostic Catheters
-
PTCA Guiding Catheters
-
Introducer Sheaths
-
-
Intravascular Imaging Catheters and Pressure Guidewires (IVUS Catheters, OCT Catheters, FFR Guidewires)
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Middle East & Africa
-
Frequently Asked Questions About This Report
b. The global interventional cardiology devices market size was estimated at USD 12.91 billion in 2022 and is expected to reach USD 13.75 billion in 2023.
b. The global interventional cardiology devices market is expected to grow at a compound annual growth rate of 7.2% from 2023 to 2030 to reach USD 22.35 billion by 2030.
b. Asia Pacific held a significant share in the industry in 2022 with a revenue share of 23.4% due to rising CAD incidence and better CAD screening, economic development, increased government investment in healthcare, favorable reimbursement in South Korea and Australia, the expansion of the healthcare infrastructure in India and China, and the rising prevalence of several CAD risk factors like poor diets, smoking, and unhealthy lifestyles.
b. Some key players operating in the interventional cardiology devices market include Abbott Laboratories; Medtronic; Boston Scientific; Terumo; B. Braun; BIOTRONIK; Biosensors International; Koninklijke Philips N.V.; Teleflex Medical; Meril Life Sciences; Merit Medical Systems; Becton, Dickinson and Company (BD)
b. Key factors that are driving the market growth include an increase in government initiatives in mature markets, rising initiatives by competitors, rising adoption of new generation devices, increasing prevalence of Coronary Artery Disease (CAD), and a rise in patient volume.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





