- Home
- »
- Clinical Diagnostics
- »
-
Point-of-Care Molecular Diagnostics Market Report, 2030GVR Report cover
![Point-of-Care Molecular Diagnostics Market Size, Share & Trends Report]()
Point-of-Care Molecular Diagnostics Market Size, Share & Trends Analysis Report By Application (Infectious Diseases, Oncology, Prenatal Testing), By Technology, By Test Location, By End-use, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: 978-1-68038-886-2
- Number of Report Pages: 150
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Market Size & Trends
The global point-of-care molecular diagnostics market size was estimated at USD 8.17 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 2.5% from 2024 to 2030. Increasing prevalence of chronic and infectious diseases and genetic disorders, such as Alzheimer’s disease, Turner syndrome, and Parkinson’s disease, is expected to boost market demand during the forecast period. Unceasing improvement in the quality of products offered on a shelf is a major factor contributing to market growth. Furthermore, the growing demand for rapid tests, coupled with a significant number of new product introductions in point-of-care (POC) molecular diagnostics, is expected to propel market growth in the near future.
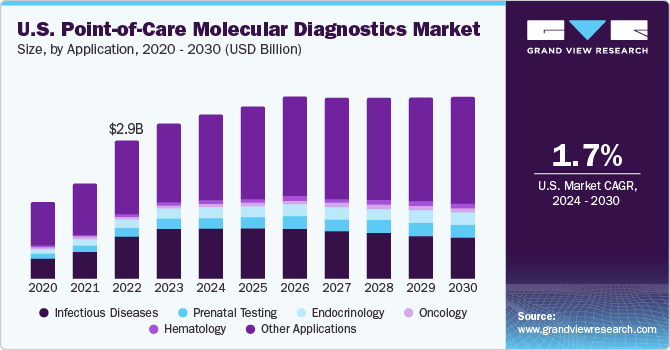
Moreover, POC molecular diagnostics in primary care settings range from simple glucose testing to complex coagulation testing. In several clinics, professionals are switching to POC testing from conventional lab testing, which helps shorten the time taken to decide whether further tests are required by avoiding delays during specimen preparation and transport. Other advantages of POC molecular diagnostics are rapid availability of results, lower costs, and better outcomes. POC molecular diagnostics market has proliferated in the past few years, especially in the U.S. and European countries, owing to increased awareness regarding various issues such as medical and organizational concerns and economic advantages of POCT.
Aging is influenced by the interaction of several environmental and genetic factors and is characterized as the single most substantial risk factor for cancer development. Based on the U.S. National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Database, 38% of women and 43% of men are estimated to develop cancer during their lifetime. Nearly two-thirds of all new cancer cases are diagnosed in people aged 65 and above, which highlights that aging can make people more vulnerable to cancer. This is where molecular diagnostics play an important role as it has a tremendous impact on the management of cancer, infectious diseases, and cardiovascular diseases. It is, therefore, vital for public health surveillance and detection. Thus, the growing geriatric population is expected to drive market growth.
Moreover, market players are continuously involved in the development of novel POC testing products to capitalize on market opportunities. For instance, in February 2023, Huwel Lifesciences designed a portable RT-PCR machine to test types of viruses. The company claimed that the test takes around 30 minutes and can detect respiratory as well as other infections using blood and gastrointestinal samples. Moreover, in April 2023, Curative, Inc. announced the spin-off of Sensible Diagnostics, focusing on the commercialization of a novel POC PCR testing platform to provide results accurately within 10 minutes.
Market Concentration & Characteristics
Advancements in technology have led to the development of smaller and more portable POC diagnostic devices. These devices could offer increased convenience and ease of use, enabling diagnostics to be performed anywhere, including resource-limited settings and remote areas. For instance, in February 2023 Co-Diagnostics started clinical evaluations for its Co-Dx PCR Home platform, developed to detect infectious diseases, and an initial COVID-19 test. The new POCT and at-home platform signifies a high degree of innovation aimed at bringing together the power of other proprietary IP and its patented Co-Primer technology, setting a new standard for compact POCT that unlocks the multiplexing potential of real-time PCR.
Furthermore, advancements in miniaturization, nanotechnology, microfluidics, and cloud-connected POC diagnostics are making molecular-level diagnostics more affordable, user-friendly, and sensitive. Smartphone-integrated readers with diverse biosensing platforms enable onsite and on-time diagnostics. 3D printing enhances POC device fabrication and performance, while flexible sensors with wireless communication allow real-time patient monitoring for preventative healthcare and disease outbreaks. These innovations signify significant progress in POC molecular diagnostics and its potential impact on healthcare, thereby driving market growth.
Moreover, growing adoption of technologically advanced testing products and rising demand for patient-centric healthcare services at POC facilities, such as retail pharmacies and clinics are further projected to drive market expansion over projected timeframe. Moreover, market players are continuously involved in development of novel POC testing products to capitalize on market opportunities. For instance, in April 2023, Curative, Inc. announced a spin-off of Sensible Diagnostics, focusing on the commercialization of a novel POC PCR testing platform to provide results within 10 minutes with high accuracy.
End-use Insights
In 2023, decentralized labs segment held the highest share of 42.7% in the global point-of-care molecular diagnostics market. Advancements in rapid detection assays are expected to bolster market growth significantly. These tests have revolutionized POC molecular diagnostics market by speeding up decision-making processes and reducing turnaround time for decentralized testing. Their ability to provide quick and accurate results has made them invaluable in various healthcare settings, driving their adoption and contributing to the overall growth of the market.Moreover,increased funding for decentralized testing is expected to boost the market. For instance, in July 2021, Q-POC, a rapid PoC diagnostic test by QuantuMDx, received funding of USD 15.54 million to facilitate its commercialization. The device would be useful in critical care settings, such as ICUs and emergency rooms, which need rapid results.
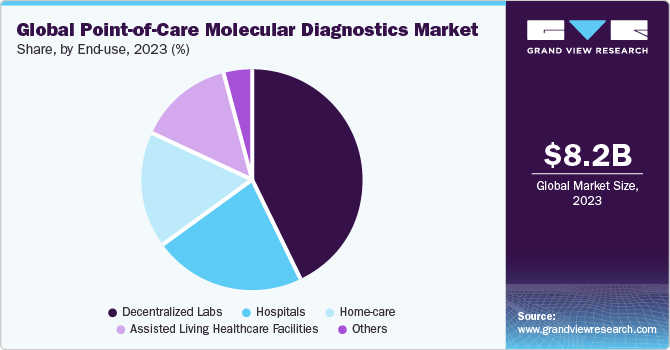
Home care segment is expected to grow at the fastest CAGR over the projected timeframe. Owing to the cost-effectiveness and comfort level of POC molecular diagnostics provided to patients at home. POC molecular diagnostics in home healthcare sector also empowers patients to address healthcare challenges at home and make decisions instantly. POCT devices are easy to use and do not, mandatorily, require any modern lab infrastructure for testing simpler target analytes in a patient’s sample. Thus, POC molecular diagnostics have potential to empower patients with self-testing abilities, specifically in terms of stigmatized health conditions, such as sexually transmitted diseases. As the emphasis of healthcare is shifting towards prevention and early detection prevention of diseases, home-based POC diagnostics is likely to witness significant growth over the study period.
Regional Insights
North America dominated the global market with a revenue share of 45.1% in 2023. The region's well-established healthcare infrastructure and technological advancements have paved the way for rapid implementation of POC molecular diagnostic technologies. The trend is driven by growing demand for faster and more accurate diagnostics, especially concerning infectious diseases like COVID-19. POC molecular tests offer the advantage of real-time results, enabling timely treatment decisions and patient management. In addition, advancements in miniaturization, AI integration, and connectivity are further enhancing the efficiency and accessibility of POC molecular diagnostics, making them a critical component of healthcare systems in North America.
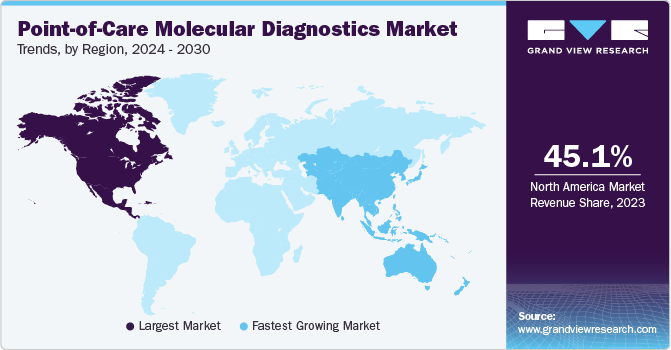
Asia Pacific is expected to register the fastest CAGR over the forecast period. Asia Pacific region is witnessing a significant rise in the adoption of POC molecular diagnostics, driven by vast and diverse healthcare landscape in the region, encompassing urban centers and remote rural areas, necessitating accessible and rapid diagnostics. POC molecular tests offer timely results without requiring centralized laboratories, improving healthcare access. Another factor driving growth is the growing burden of infectious diseases in the Asia Pacific, including COVID-19, which underscores the urgency for quick and accurate diagnostics.
Application Insights
Infectious disease segment accounted for 31.5% of the overall market in 2023. The global burden of infectious diseases is growing, leading to an increase in hospitalizations and mortality. For instance, according to WHO, HIV continues to be a major public health concern globally. Over 38.4 million people were estimated to be living with HIV in 2021 globally, representing a prevalence of about 0.7% in adults. Advanced Point-Of-Care (POC) is being developed for a broad range of infectious diseases to meet the growing demand for various testing alternatives boosting segment’s growth. For instance, in November 2022, NOWDiagnostics announced that ADEXUSDx, its 4th generation HIV test, has been developed and has received a CE mark. Moreover, affordability, ease of use, and reliability associated with POC for a variety of infectious diseases are further propelling segment growth.
The Oncology segment is estimated to emerge as the most lucrative segment of all. The globally rising prevalence of cancer represents an increasing cause for concern, as cancer is a leading cause of death and accounted for about 10 million deaths in 2020, as per WHO. The most common cancer types are lung, breast, colon & rectum, and prostate cancers; and POC tests are being rapidly adopted for enabling mass screening as well as early detection for a broad range of cancer types. Furthermore, presence of a large number of government-funded testing programs, such as the National Breast and Cervical Cancer Early Detection Program, is expected to drive market growth.
Technology Insights
The PCR-based segment held the largest share of 65.4% in the global market in 2023. PCR-based technology is the most trusted and conventional gold standard technology for amplification of DNA material to perform POC molecular diagnosis. PCR is the foremost and simplest procedure performed before any molecular diagnostic investigation involving genetic material. An increase in applications of POC molecular diagnostics in fields of drug discovery and development, cancer research, pharmacogenomics, and acquired immunodeficiency syndrome directly boosts demand for PCR technologies. Therefore, PCR-based segment held a significant share due to the advantages and varied applications of multiplex PCR over conventional PCR methodologies.
The genetic sequencing-based POC molecular diagnostics segment is anticipated to register the fastest CAGR during the forecast period. Genetic sequencing-based technology enables real-time analysis of genetic material, facilitating early disease detection and personalized treatment decisions at point of care. Market growth is attributed to increasing demand for rapid and accurate diagnostic tests and rising prevalence of infectious diseases. Furthermore, advancements in sequencing techniques have led to increased adoption in various medical settings, driving market expansion. Moreover, growing demand for personalized medicine and expanding applications of genetic sequencing are driving segment growth.
Test Location Insights
In 2023, OTC diagnostic held the highest share of 51.8% in the global market. OTC diagnostic products offer early diagnosis and constant patient monitoring as well as curb costs associated with healthcare establishments and practitioners. With an increasing demand for POC molecular diagnostics, the need for comprehensive testing platforms is growing to assist patients in conducting diagnosis without the guidance of practitioners. Several market players are launching novel kits for the diagnosis of various infectious diseases. For instance, in March 2023, Lucira Health announced the launch of first and only at-home COVID-19 and flu tests in the U.S. Such, initiatives are spurring segment growth.
Point of care test location segment is estimated to register fastest growth during forecast period. POC offers tests with quick results and portability. Molecular testing identifies an infectious agent's genetic material and is a type of POC test kits that is expanding at fastest rate. Numerous tests are being used outside of hospitals since they are quick, portable, and affordable. Abbott, Roche Diagnostics, and Cepheid, for example, have already established their presence in this sector. Growing interest in developing molecular diagnostic platforms in PoC settings is driving various companies to design assays and molecular diagnostic systems for near-patient testing. For instance, in April 2023, Truenat H3N2/H1N1, the initial PoC Real-Time Polymerase Chain Reaction (PCR) test that assists in proven identification of influenza infections, has been made available, according to Mobilo Diagnostics.
Key Companies & Market Share Insights
Key players are focusing on new product development and seeking regulatory approvals at a rapid pace. In addition, numerous market players are undertaking several strategic initiatives such as partnerships, collaborations, mergers, acquisitions, and expansion.
-
In January 2023, F. Hoffmann-La Roche Ltd introduced a COVID-19 RT-PCR test for the fast-spreading COVID-19 Omicron subvariant, XBB.1.5.
-
In January 2023, ReadyGo Diagnostics and Gemina Laboratories Ltd. entered into a partnership to develop Mycobacterium Tuberculosis saliva-based tests.
Key Point-of-care Molecular Diagnostics Companies:
- F. Hoffmann-La Roche AG
- Abbott
- QIAGEN
- Bayer AG
- Nova Biomedical
- Danaher
- Nipro Diagnostics
- Bio-Rad Laboratories, Inc.
- Agilent Technologies, Inc.
- bioMérieux
- OraSure Technologies
- Abaxis
Point-of-Care Molecular Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 8.70 billion
Revenue forecast in 2030
USD 10.12 billion
Growth rate
CAGR of 2.5% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Report updated
December 2023
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Application, technology, test location, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Abbott; Bayer AG; F. Hoffmann-La Roche AG; Nova Biomedical; QIAGEN; Nipro Diagnostics; Danaher; Bio-Rad Laboratories, Inc.; bioMérieux; Agilent Technologies, Inc.; Abaxis; OraSure Technologies.
Customization scope
Free report customization (equivalent up to 8 analyst working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Point-of-Care Molecular Diagnostics Market Report Segmentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global point-of-care molecular diagnostics market based on application, technology, test location, end-use, and region:
-
Technology Outlook (Revenue, USD Million, 2018 - 2030)
-
PCR-based
-
Genetic Sequencing-based
-
Hybridization-based
-
Microarray-based
-
-
Application Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Infectious Diseases
-
HIV POC
-
Clostridium difficile POC
-
HBV POC
-
Pneumonia or Streptococcus associated infections
-
Respiratory syncytial virus (RSV) POC
-
HPV POC
-
Influenza/Flu POC
-
HCV POC
-
MRSA POC
-
TB and drug-resistant TB POC
-
HSV POC
-
Other Infectious Diseases
-
-
Oncology
-
Hematology
-
Prenatal Testing
-
Endocrinology
-
Other Applications
-
-
Test Location Outlook (Revenue, USD Million, 2018 - 2030)
-
OTC
-
POC
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Decentralized Labs
-
Hospitals
-
Home-care
-
Assisted Living Healthcare Facilities
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
U.K.
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global point-of-care molecular diagnostics market size was estimated at USD 8.17 billion in 2023 and is expected to reach USD 8.70 billion in 2024.
b. The global point-of-care molecular diagnostics market is expected to grow at a compound annual growth rate of 2.5% from 2024 to 2030 to reach USD 10.12 billion by 2030.
b. Decentralised labs segment dominated the point-of-care molecular diagnostics market with a share of 42.66% in 2023. Advancements in rapid detection assays are expected to bolster market growth significantly. Their ability to provide quick and accurate results has made them invaluable in various healthcare settings, driving their adoption and contributing to the overall growth of market.
b. Some key players operating in the point-of-care molecular diagnostics market include Abbott Laboratories, Bayer Healthcare; F. Hoffmann-La Roche AG; Becton, Dickinson and Company; BioMerieux; Bio-Rad Laboratories, Inc.; Cepheid, Inc.; Danaher Corporation; and Johnson & Johnson Services, Inc.
b. Key factors that are driving the market growth include high quality of test results in very short time, development of accurate & faster testing methods, and offering convenient diagnostic approach for physicians.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





