- Home
- »
- Clinical Diagnostics
- »
-
Point Of Care Diagnostics Market Size, Industry Report, 2030GVR Report cover
![Point Of Care Diagnostics Market Size, Share & Trends Report]()
Point Of Care Diagnostics Market Size, Share & Trends Analysis Report By Product (Infectious Diseases, Glucose Testing, Cardiac Markers), By End-use (Clinics, Home, Hospitals), By Region, And Segment Forecasts, 2024 - 2030
- Report ID: 978-1-68038-046-0
- Number of Report Pages: 295
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Point Of Care Diagnostics Market Trends
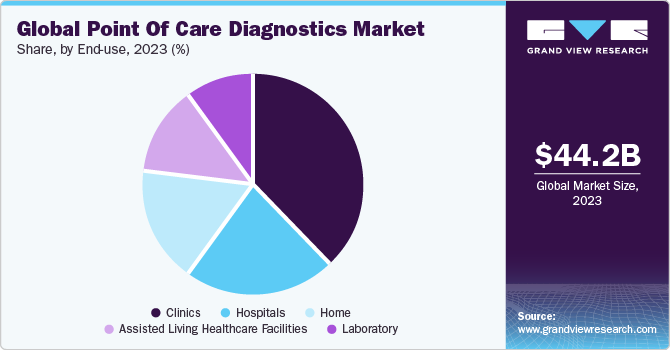
The global point of care diagnostics market size was valued at USD 44.24 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.1% from 2024 to 2030. The increasing geriatric population and the ability of point of care (POC) diagnostic tests to provide immediate results is expected to fuel market demand. Furthermore, the increasing adaptability of mobile diagnostic devices in middle-income countries is also one of the key factors for the growth of point of care testing. In addition, a rise in funding from government & private institutions is a key trend driving the growth of this market. For instance, in September 2022, Unitaid announced that it would invest USD 30 million to accelerate the introduction of advanced diagnostic technologies, which would further enhance screening efforts and expand testing availability at lower tiers of the healthcare system.
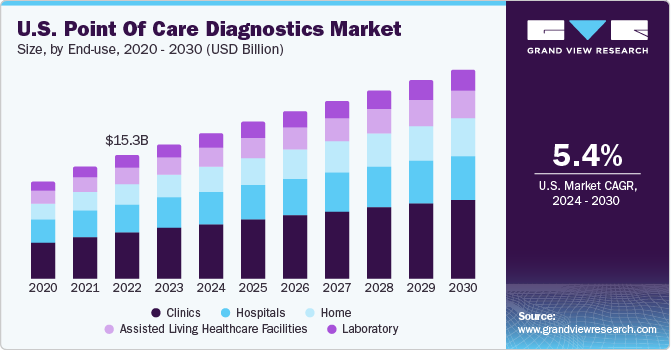
The increasing prevalence of genetic disorders is likely to drive the PoC diagnostics market in the coming years. According to a report published by the CDC, congenital heart defects were the most common birth defects in the U.S., affecting approximately 1% of births every year. Early detection of genetic variations via prenatal testing can help in rapid disease diagnosis, prevention, and selection of suitable treatment. Changing lifestyles and environmental factors are increasing the incidence of genetic diseases. The incidence of breast and ovarian cancers is increasing in many regions. For instance, according to the Breast Cancer Research Foundation, in 2023, approximately 300,590 people are expected to be diagnosed with breast cancer in the U.S. Thus, the growing incidence of various diseases is likely to boost the market over the forecast period.
Moreover, the geriatric population is rapidly increasing across the globe. According to a UN report, in 2020, there were about 727 million people aged 65 & above globally. In addition, the number of individuals aged 80 and above is projected to double by 2050, which is over 1.5 billion. Aging has become a substantial risk factor for numerous diseases, including obesity and diabetes, which, in turn, significantly increase the risk of infectious diseases. The geriatric population is susceptible to numerous diseases such as cancer, cardiovascular diseases, obesity, neurological disorders, and diabetes. Thus, the growing geriatric population globally is anticipated to be a high-impact rendering driver of the market.
Market Concentration & Characteristics
Market growth stage is high, and the pace of market growth is accelerating. The market is characterized by a high degree of innovation owing to rapid technological advancements and the launch of novel products by key players in the market. For instance, in February 2023, Huwel Lifesciences designed a portable RT-PCR machine to test types of viruses. The company claimed that the test takes around 30 minutes and can be used to detect respiratory and other infections using blood and gastrointestinal samples.
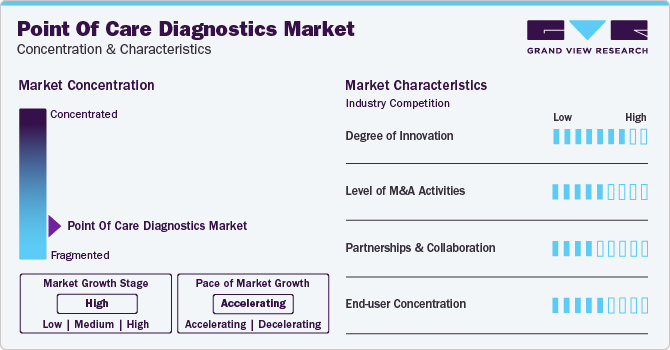
The market is also characterized by a high level of strategic initiatives such as collaborations, partnerships, and merger & acquisition (M&A) by leading players. For instance, in March 2023, Werfen acquired Immucor, Inc. to establish a strong presence in Specialized Diagnostics. The deal was valued at USD 2 billion. Similarly, in May 2023, Siemens Healthcare GmbH partnered with Unilabs, with an agreed value exceeding USD 200 million. Within the partnership, Unilabs will purchase 400 laboratory analyzers to improve patient care infrastructure. Operating players in the market leverage these strategies to increase their product capabilities and promote the reach of their offerings.
End-user concentration is a significant factor in the point of care diagnostics industry. Since there are several end-user that are driving demand for point of care diagnostic solutions. The concentration of demand in clinics and hospitals creates opportunities for companies that focus on developing point of care diagnostics. Moreover, point of care diagnostic products for home care are anticipated to witness widespread adoption due to the cost-effectiveness and comfort they offer in conducting diagnostic tests at home for patients.
Product Insights
Infectious disease led the market and accounted for 27.8% of global revenue share in 2023. Growth of the segment is attributed to increasing demand for rapid tests, which has encouraged industry players to deliver point of care solutions to decentralized regions and launch innovative solutions. For instance, in May 2023, Sensible Diagnostics announced plans to launch a POC PCR instrument that can perform PCR in 10 minutes by 2024, with an initial focus on infectious diseases. Moreover, Abbott has announced the launch of ID NOW, the world's fastest molecular POC test, which offers COVID-19 results in 13 minutes and can be used in a range of dispersed healthcare settings, such as doctor offices & urgent care clinics. Moreover, the implementation of government initiatives aimed at curbing healthcare expenditure by restricting the growth of the incidence of infectious diseases will serve this industry as a driver.
The cancer segment is expected to register the fastest CAGR during the forecast period. The growing use of markers in cancer diagnostics is the primary factor likely to boost the cancer markers segment during the forecast period. The rising global prevalence of cancer is driving the need for early disease detection, and is also expected to propel the market. For instance, according to Cancer.org, approximately 1.9 million new cases of cancer were diagnosed in the U.S. in 2021, with 608,570 cancer deaths. Moreover, key players in the market are introducing novel products to meet untapped opportunities in the market. For instance, in October 2022, F. Hoffman-La Roche Ltd., was approved for the first companion diagnostic by the U.S. FDA to detect patients with HER2 low metastatic breast cancer eligible for ENHERTU.
End-use Insights
The clinic end-use segment led the market and accounted for the highest revenue share in 2023. Point of care diagnostics in primary care settings ranges from simple glucose testing to complex coagulation testing. In several clinics, professionals are switching to point of care diagnostics from conventional lab testing, and this helps shorten the time taken to decide whether further tests are required by avoiding delays during specimen preparation and transport. Other advantages of point of care diagnostics are rapid availability of results, lower costs, and better outcomes. The market has grown rapidly in the past few years, especially in the U.S. and European countries, owing to increased awareness regarding various issues such as medical & organizational concerns and economic advantages of point of care diagnostics.
Home sector is projected to witness the highest growth rate over the forecast period owing to the comfort level and cost-effectiveness of point of care diagnostics provided to patients at home. POC in the home healthcare sector also allows patients to address healthcare challenges at home and make decisions instantly. Moreover, with the rise in the aging population, which is more susceptible to chronic diseases, the demand for healthcare services is likely to show steady growth in the coming years. This will boost the home healthcare sector, which can improve the overall access and reach to medical care while helping avoid unnecessary visits, hospital admissions, and readmissions, as well as time & costs associated with traveling to meet healthcare providers.
Regional Insights
North America dominated the market and accounted for a 42.9% share in 2023. The growing geriatric population and the presence of higher healthcare expenditure are some key trends contributing to its largest share. Furthermore, technological advancements such as the launch of miniaturized diagnostic equipment offering accurate and rapid results and increasing market penetration of Picture Archiving and Communication Systems (PACS) and Electronic Medical Records (EMRs) are anticipated to propel market growth during the forecast period. Moreover, the presence of key players such as Abbott, BIOMERIEUX, BD, Siemens Healthineers AG, QIAGEN, Quidel Corporation, and Quest Diagnostics is positively influencing market growth. For instance, in April 2023, Abbott announced that the U.S. FDA cleared reader for FreeStyle Libre 3 integrated continuous glucose monitoring system.
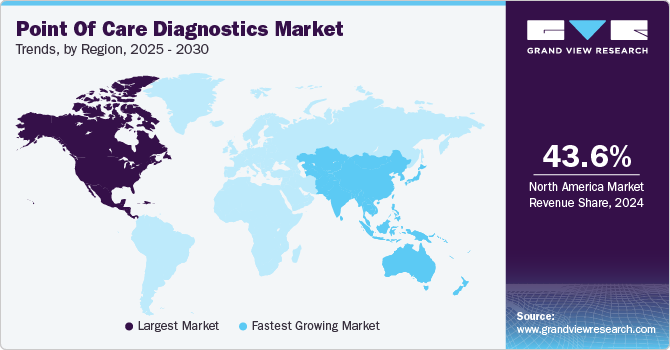
Asia Pacific is anticipated to witness significant growth in point of care diagnostics market. Development of healthcare infrastructure coupled with a higher prevalence of chronic and targeted diseases, such as diabetes and cancer along with infectious conditions including HIV, syphilis, and RSV, are expected to drive market in Asia Pacific countries. Adoption of POC diagnostics in operating rooms, emergency rooms, intensive care units, path labs, and hospitals is expected to rise owing to early and efficient results. Moreover, presence of a strong product pipeline pertaining to infectious and cardiac markers segment is estimated to enhance market through to 2030.
Furthermore, rise in adoption of miniaturized models and measures adopted for reducing stays in hospitals and clinics especially in India are expected to fuel the demand for India point of care diagnostic industry.Moreover, rapid technological advancements, high prevalence of chronic & infectious diseases, and continuous efforts by local companies as well as organizations are driving the market in India. For instance, in January 2023, Cipla Limited, based in India, launched Cippoint, a POC testing device that offers a wide array of testing parameters, such as diabetes, cardiac markers, fertility, infectious diseases, inflammation, thyroid function, coagulation markers, and metabolic markers.
Key Point Of Care Diagnostics Company Insights
Some of the key players operating in the market include Abbott; F. Hoffmann-La Roche Ltd., Inc.; Siemens Healthcare GmbH, and Danaher Corporation. Established players focus majorly on innovation & technology advancements to develop cutting-edge diagnostic solutions and partner with emerging players to leverage their technology. Mature players also have a strong global presence with a diverse portfolio of PoC products and a well-established brand reputation which gives them a competitive edge.
Emerging players however focus on launching products in limited countries and then expanding regionally. Some operating strategies also include strategic partnerships, acquisitions, or collaborations to enhance their capabilities and market presence. Additionally, these players may be more flexible and agile than established players in terms of responding and changing to market needs and demands, allowing them to quickly adapt and develop new technologies.
Key Point Of Care Diagnostics Companies:
The following are the leading companies in the point of care diagnostics market. These companies collectively hold the largest market share and dictate industry trends. Financials, strategy maps & products of these point of care diagnostics companies are analyzed to map the supply network.
- F. Hoffmann-La Roche Ltd.
- Qiagen
- Danaher Corporation
- Becton Dickinson (BD)
- bioMérieux
- Abbott
- Siemens Healthcare GmbH
- Werfen
- Nova Biomedical
- Trividia Health, Inc.
- QuidelOrtho Corporation
- Trinity Biotech
- Sekisui Diagnostics
- Orasure Technologies, Inc.
- Spectral Medical, Inc.
- EKF Diagnostics Holdings plc.
- Anbio Biotechnology Co., Ltd.
- AccuBioTech Co., Ltd
- ALPHA LABORATORIES.
Recent Developments
-
In October 2023, QIAGEN gained CE certification for IVD kit and automated testing platform, NeuMoDx. This initiative positively impacted the company’s revenue and share in the market
-
In May 2023, Danaher Corporation, introduced Dxl 9000 Access Immunoassay Analyzer, which can run up to 215 test per hour. This initiative expanded the company’s point of care diagnostics offering
-
In May 2023, BD announced 510k clearance for the BD Kiestra Methicillin-resistant Staphylococcus aureus (MRSA) imaging application that uses AI software. This launch would increase the turnaround time of tests results as the software eliminates the time-intensive task of determining bacterial growth using petri dishes
-
In March 2023, Trinity Biotech, TrinScreen HIV was included in the New Kenyan HIV Testing Algorithm. This initiative was aimed at increasing access to the product for HIV patients
-
In March 2023, F. Hoffman-La Roche Ltd entered into a strategic partnership with Eli Lilly to develop former’s Elecsys Amyloid Plasma Panel (EAPP). This collaboration would benefit both the companies and would target an important area of unmet medical need as this panel has the potential to streamline patients journey from diagnosis to treatment and treatment management
Point Of Care Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 47.8 billion
Revenue forecast in 2030
USD 68.5 billion
Growth Rate
CAGR of 6.1% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada, Germany; U.K.; France; Italy; Spain; Russia; Denmark; Sweden; Norway; Japan; China; India; South Korea; Australia; Thailand; Brazil Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
F. Hoffmann-La Roche Ltd.; Qiagen; Danaher Corporation; Becton Dickinson (BD); bioMérieux; Abbott; Siemens Healthcare GmbH; Werfen; Nova Biomedical; Trividia Health, Inc.; QuidelOrtho Corporation; Trinity Biotech; Sekisui Diagnostics; Orasure Technologies, Inc.; Spectral Medical, Inc.; EKF Diagnostics Holdings Plc.; Anbio Biotechnology Co., Ltd.; AccuBioTech Co., Ltd.; ALPHA LABORATORIES
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Point Of Care Diagnostics Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global point of care diagnostics market report based on product, end use, and region.
-
Product Outlook (Revenue, USD Billion, 2018 - 2030)
-
Glucose Testing
-
Hb1Ac Testing
-
Coagulation Testing
-
Fertility/Pregnancy
-
Infectious Disease
-
HIV POC
-
Clostridium Difficile POC
-
HBV POC
-
Pneumonia or Streptococcus Associated Infections
-
Respiratory Syncytial Virus (RSV) POC
-
HPV POC
-
Influenza/Flu POC
-
HCV POC
-
MRSA POC
-
TB and Drug-resistant TB POC
-
HSV POC
-
COVID-19
-
Other Infectious Diseases
-
-
Cardiac Markers
-
Thyroid Stimulating Hormone
-
Hematology
-
Primary Care Systems
-
Decentralized Clinical Chemistry
-
Feces
-
Lipid Testing
-
Cancer Marker
-
Blood Gas/Electrolytes
-
Ambulatory Chemistry
-
Drug of Abuse (DOA) Testing
-
Autoimmune Diseases
-
Urinalysis/Nephrology
-
-
End-use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Clinics
-
Hospitals
-
Home
-
Assisted Living Healthcare Facilities
-
Laboratory
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
U.K.
-
France
-
Italy
-
Spain
-
Russia
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global point of care diagnostics market size was estimated at USD 44.24 billion in 2023 and is expected to reach USD 47.8 billion in 2024.
b. The global point of care diagnostics market is expected to grow at a compound annual growth rate of 6.1% from 2024 to 2030 to reach USD 68.5 billion by 2030.
b. The infectious disease segment dominated the point of care diagnostics market with a share of 27.85% in 2023. The rising prevalence of HIV/AIDS, influenza, RSV, and other diseases is leading to an increase in the number of people being diagnosed.
b. Some key players operating in the POC diagnostics market include F. Hoffmann-La Roche Ltd.; Abbott; Siemens; Danaher; bioMérieux SA; Johnson and Johnson; Abaxis, Inc.; QIAGEN; Nova Biomedical; Trividia Health, Inc.; Quidel Corporation; OraSure Technologies Inc.; Becton Dickinson and Company; Spectral Medical, Inc.; and Nipro.
b. Key factors that are driving the point of care diagnostics market growth include robust government initiatives, the presence of favorable regulations, and the advent of next-generation point of care diagnostics technologies.
Table of Contents
Chapter 1. Methodology and Scope
1.1. Market Segmentation & Scope
1.2. Segment Definitions
1.2.1. Product
1.2.2. End Use
1.2.3. Regional scope
1.2.4. Estimates and forecasts timeline
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased database
1.4.2. GVR’s internal database
1.4.3. Secondary sources
1.4.4. Primary research
1.4.5. Details of primary research
1.4.5.1. Data for primary interviews in North America
1.4.5.2. Data for primary interviews in Europe
1.4.5.3. Data for primary interviews in Asia Pacific
1.4.5.4. Data for primary interviews in Latin America
1.4.5.5. Data for Primary interviews in MEA
1.5. Information or Data Analysis
1.5.1. Data analysis models
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.2. Approach 1: Commodity flow approach
1.7.3. Volume price analysis (Model 2)
1.7.4. Approach 2: Volume price analysis
1.8. List of Secondary Sources
1.9. List of Primary Sources
1.10. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.2.1. Product outlook
2.2.2. End Use outlook
2.2.3. Regional outlook
2.3. Competitive Insights
Chapter 3. Point Of Care Diagnostics Market Variables, Trends & Scope
3.1. Market Lineage Outlook
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook
3.2. Market Dynamics
3.2.1. Market driver analysis
3.2.1.1. Introduction of CLIA waived tests
3.2.1.2. Rise in funding from government and private institutions
3.2.1.3. Growing geriatric population base
3.2.1.4. Growing prevalence of target diseases
3.2.1.5. Growing demand for home healthcare and the introduction of advance technology enabled products
3.2.2. Market restraint analysis
3.2.2.1. High procedure costs coupled with limited adoption of POC devices in certain emerging regions
3.2.2.2. Presence of ambiguous regulatory as well as reimbursement frame work for primary care setting
3.2.3. Market challenge analysis
3.2.3.1. POC device pose challenges in maintaining the quality standards
3.2.4. Market opportunity analysis
3.2.4.1. Networking & remote integration of POC diagnostics products
3.2.4.1.1. Smartphone orientation
3.2.4.1.2. Embedded vision based solutions
3.2.4.1.3. Digital technologies
3.3. Point Of Care Diagnostics Market Analysis Tools
3.3.1. Industry Analysis - Porter’s Five Forces
3.3.1.1. Supplier power
3.3.1.2. Buyer power
3.3.1.3. Substitution threat
3.3.1.4. Threat of new entrant
3.3.1.5. Competitive rivalry
3.3.2. PESTEL Analysis
3.3.2.1. Political landscape
3.3.2.2. Technological landscape
3.3.2.3. Economic landscape
3.4. Pricing Analysis, By Product
3.4.1. Glucose Testing
3.4.2. Hb1Ac Testing
3.4.3. Coagulation Testing
3.4.4. Fertility/Pregnancy
3.4.5. Infectious Disease
3.4.6. HIV POC
3.4.7. Clostridium Difficile POC
3.4.8. HBV POC
3.4.9. Pneumonia or Streptococcus Associated Infections
3.4.10. Respiratory Syncytial Virus (RSV) POC
3.4.11. HPV POC
3.4.12. Influenza/Flu POC
3.4.13. HCV POC
3.4.14. MRSA POC
3.4.15. TB and Drug-resistant TB POC
3.4.16. HSV POC
3.4.17. COVID-19
3.4.18. Other Infectious Diseases
3.4.19. Cardiac Markers
3.4.20. Thyroid Stimulating Hormone
3.4.21. Hematology
3.4.22. Primary Care Systems
3.4.23. Decentralized Clinical Chemistry
3.4.24. Feces
3.4.25. Lipid Testing
3.4.26. Cancer Marker
3.4.27. Blood Gas/Electrolytes
3.4.28. Ambulatory Chemistry
3.4.29. Drug of Abuse (DOA) Testing
3.4.30. Autoimmune Diseases
3.4.31. Urinalysis/Nephrology
Chapter 4. Point Of Care Diagnostics Market: Product Estimates & Trend Analysis
4.1. Product Market Share, 2023 & 2030
4.2. Segment Dashboard
4.3. Global Point Of Care Diagnostics Market by Product Outlook
4.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
4.4.1. Glucose Testing
4.4.1.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.2. Hb1Ac Testing
4.4.2.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.3. Coagulation Testing
4.4.3.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.4. Fertility/Pregnancy Testing
4.4.4.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5. Infectious Disease
4.4.5.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.2. HIV POC
4.4.5.2.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.3. Clostridium Difficile POC
4.4.5.3.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.4. HBV POC
4.4.5.4.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.5. Pneumonia or Streptococcus Associated Infections
4.4.5.5.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.6. Respiratory Syncytial Virus (RSV) POC
4.4.5.6.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.7. HPV POC
4.4.5.7.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.8. Influenza/Flu POC
4.4.5.8.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.9. HCV POC
4.4.5.9.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.10. MRSA POC
4.4.5.10.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.11. TB and drug resistant TB POC
4.4.5.11.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.12. HSV POC
4.4.5.12.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.13. COVID-19
4.4.5.13.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.5.14. Other infectious diseases
4.4.5.14.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.6. Cardiac Markers
4.4.6.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.7. Thyroid Stimulating Hormone
4.4.7.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.8. Hematology
4.4.8.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.9. Primary Care Systems
4.4.9.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.10. Decentralized Clinical Chemistry
4.4.10.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.11. Feces
4.4.11.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.12. Lipid Testing
4.4.12.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.13. Cancer Marker
4.4.13.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.14. Blood Gas/Electrolytes
4.4.14.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.15. Ambulatory Chemistry
4.4.15.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.16. Drug of Abuse (DOA) Testing
4.4.16.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.17. Autoimmune diseases
4.4.17.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
4.4.18. Urinalysis/Nephrology
4.4.18.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
Chapter 5. Point Of Care Diagnostics Market: End Use Estimates & Trend Analysis
5.1. End Use Market Share, 2023 & 2030
5.2. Segment Dashboard
5.3. Global Point Of Care Diagnostics Market by End Use Outlook
5.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
5.4.1. Clinics
5.4.1.1. Market estimates and forecasts 2018 to 2030 (USD billion)
5.4.1.2. Pharmacy & Retail Clinics
5.4.1.2.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
5.4.1.3. Physician Office
5.4.1.3.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
5.4.1.4. Urgent Care Clinics
5.4.1.4.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
5.4.1.5. Non-practice Clinics
5.4.1.5.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
5.4.2. Hospitals
5.4.2.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
5.4.3. Home
5.4.3.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
5.4.4. Assisted Living Healthcare Facilitates
5.4.4.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
5.4.5. Laboratory
5.4.5.1. Market estimates and forecasts 2018 to 2030 (USD Billion)
Chapter 6. Point Of Care Diagnostics Market: Regional Estimates & Trend Analysis
6.1. Regional Market Share Analysis, 2023 & 2030
6.2. Regional Market Dashboard
6.3. Global Regional Market Snapshot
6.4. Market Size, & Forecasts Trend Analysis, 2018 to 2030:
6.5. North America
6.5.1. U.S.
6.5.1.1. Key country dynamics
6.5.1.2. Regulatory framework/ reimbursement structure
6.5.1.3. Competitive scenario
6.5.1.4. U.S. market estimates and forecasts 2018 to 2030 (USD Billion)
6.5.2. Canada
6.5.2.1. Key country dynamics
6.5.2.2. Regulatory framework/ reimbursement structure
6.5.2.3. Competitive scenario
6.5.2.4. Canada market estimates and forecasts 2018 to 2030 (USD Billion)
6.6. Europe
6.6.1. Germany
6.6.1.1. Key country dynamics
6.6.1.2. Regulatory framework/ reimbursement structure
6.6.1.3. Competitive scenario
6.6.1.4. Germany market estimates and forecasts 2018 to 2030 (USD Billion)
6.6.2. UK
6.6.2.1. Key country dynamics
6.6.2.2. Regulatory framework/ reimbursement structure
6.6.2.3. Competitive scenario
6.6.2.4. UK market estimates and forecasts 2018 to 2030 (USD Billion)
6.6.3. France
6.6.3.1. Key country dynamics
6.6.3.2. Regulatory framework/ reimbursement structure
6.6.3.3. Competitive scenario
6.6.3.4. France market estimates and forecasts 2018 to 2030 (USD Billion)
6.6.4. Italy
6.6.4.1. Key country dynamics
6.6.4.2. Regulatory framework/ reimbursement structure
6.6.4.3. Competitive scenario
6.6.4.4. Italy market estimates and forecasts 2018 to 2030 (USD Billion)
6.6.5. Spain
6.6.5.1. Key country dynamics
6.6.5.2. Regulatory framework/ reimbursement structure
6.6.5.3. Competitive scenario
6.6.5.4. Spain market estimates and forecasts 2018 to 2030 (USD Billion)
6.6.6. Russia
6.6.6.1. Key country dynamics
6.6.6.2. Regulatory framework/ reimbursement structure
6.6.6.3. Competitive scenario
6.6.6.4. Russia market estimates and forecasts 2018 to 2030 (USD Billion)
6.6.7. Norway
6.6.7.1. Key country dynamics
6.6.7.2. Regulatory framework/ reimbursement structure
6.6.7.3. Competitive scenario
6.6.7.4. Norway market estimates and forecasts 2018 to 2030 (USD Billion)
6.6.8. Sweden
6.6.8.1. Key country dynamics
6.6.8.2. Regulatory framework/ reimbursement structure
6.6.8.3. Competitive scenario
6.6.8.4. Sweden market estimates and forecasts 2018 to 2030 (USD Billion)
6.6.9. Denmark
6.6.9.1. Key country dynamics
6.6.9.2. Regulatory framework/ reimbursement structure
6.6.9.3. Competitive scenario
6.6.9.4. Denmark market estimates and forecasts 2018 to 2030 (USD Billion)
6.7. Asia Pacific
6.7.1. Japan
6.7.1.1. Key country dynamics
6.7.1.2. Regulatory framework/ reimbursement structure
6.7.1.3. Competitive scenario
6.7.1.4. Japan market estimates and forecasts 2018 to 2030 (USD Billion)
6.7.2. China
6.7.2.1. Key country dynamics
6.7.2.2. Regulatory framework/ reimbursement structure
6.7.2.3. Competitive scenario
6.7.2.4. China market estimates and forecasts 2018 to 2030 (USD Billion)
6.7.3. India
6.7.3.1. Key country dynamics
6.7.3.2. Regulatory framework/ reimbursement structure
6.7.3.3. Competitive scenario
6.7.3.4. India market estimates and forecasts 2018 to 2030 (USD Billion)
6.7.4. South Korea
6.7.4.1. Key country dynamics
6.7.4.2. Regulatory framework/ reimbursement structure
6.7.4.3. Competitive scenario
6.7.4.4. South Korea market estimates and forecasts 2018 to 2030 (USD Billion)
6.7.5. Australia
6.7.5.1. Key country dynamics
6.7.5.2. Regulatory framework/ reimbursement structure
6.7.5.3. Competitive scenario
6.7.5.4. Australia market estimates and forecasts 2018 to 2030 (USD Billion)
6.7.6. Thailand
6.7.6.1. Key country dynamics
6.7.6.2. Regulatory framework/ reimbursement structure
6.7.6.3. Competitive scenario
6.7.6.4. Thailand market estimates and forecasts 2018 to 2030 (USD Billion)
6.8. Latin America
6.8.1. Brazil
6.8.1.1. Key country dynamics
6.8.1.2. Regulatory framework/ reimbursement structure
6.8.1.3. Competitive scenario
6.8.1.4. Brazil market estimates and forecasts 2018 to 2030 (USD Billion)
6.8.2. Mexico
6.8.2.1. Key country dynamics
6.8.2.2. Regulatory framework/ reimbursement structure
6.8.2.3. Competitive scenario
6.8.2.4. Mexico market estimates and forecasts 2018 to 2030 (USD Billion)
6.8.3. Argentina
6.8.3.1. Key country dynamics
6.8.3.2. Regulatory framework/ reimbursement structure
6.8.3.3. Competitive scenario
6.8.3.4. Argentina market estimates and forecasts 2018 to 2030 (USD Billion)
6.9. MEA
6.9.1. South Africa
6.9.1.1. Key country dynamics
6.9.1.2. Regulatory framework/ reimbursement structure
6.9.1.3. Competitive scenario
6.9.1.4. South Africa market estimates and forecasts 2018 to 2030 (USD Billion)
6.9.2. Saudi Arabia
6.9.2.1. Key country dynamics
6.9.2.2. Regulatory framework/ reimbursement structure
6.9.2.3. Competitive scenario
6.9.2.4. Saudi Arabia market estimates and forecasts 2018 to 2030 (USD Billion)
6.9.3. UAE
6.9.3.1. Key country dynamics
6.9.3.2. Regulatory framework/ reimbursement structure
6.9.3.3. Competitive scenario
6.9.3.4. UAE market estimates and forecasts 2018 to 2030 (USD Billion)
6.9.4. Kuwait
6.9.4.1. Key country dynamics
6.9.4.2. Regulatory framework/ reimbursement structure
6.9.4.3. Competitive scenario
6.9.4.4. Kuwait market estimates and forecasts 2018 to 2030 (USD Billion)
Chapter 7. Competitive Landscape
7.1. Recent Developments & Impact Analysis, By Key Market Participants
7.2. Company/Competition Categorization
7.3. Vendor Landscape
7.3.1. List of key distributors and channel partners
7.3.1.1. Abbott
7.3.1.2. Alere Inc.
7.3.1.3. F. Hoffmann-La Roche Ltd.
7.3.1.4. Siemens Healthcare GmbH
7.3.1.5. Danaher Corporation
7.3.1.6. Werfen
7.3.1.7. BD
7.3.2. Key customers
7.3.3. Key company market share analysis, 2023
7.3.4. Company Profiles
7.3.4.1. F. Hoffmann-La Roche Ltd.
7.3.4.1.1. Company overview
7.3.4.1.2. Financial performance
7.3.4.1.3. Product benchmarking
7.3.4.1.4. Strategic initiatives
7.3.4.2. Qiagen
7.3.4.2.1. Company overview
7.3.4.2.2. Financial performance
7.3.4.2.3. Product benchmarking
7.3.4.2.4. Strategic initiatives
7.3.4.3. Danaher Corporation
7.3.4.3.1. Company overview
7.3.4.3.2. Financial performance
7.3.4.3.3. Product benchmarking
7.3.4.3.4. Strategic initiatives
7.3.4.4. Becton Dickinson (BD)
7.3.4.4.1. Company overview
7.3.4.4.2. Financial performance
7.3.4.4.3. Product benchmarking
7.3.4.4.4. Strategic initiatives
7.3.4.5. bioMérieux
7.3.4.5.1. Company overview
7.3.4.5.2. Financial performance
7.3.4.5.3. Product benchmarking
7.3.4.5.4. Strategic initiatives
7.3.4.6. Abbott
7.3.4.6.1. Company overview
7.3.4.6.2. Financial performance
7.3.4.6.3. Product benchmarking
7.3.4.6.4. Strategic initiatives
7.3.4.7. Siemens Healthcare GmbH
7.3.4.7.1. Company overview
7.3.4.7.2. Financial performance
7.3.4.7.3. Product benchmarking
7.3.4.7.4. Strategic initiatives
7.3.4.8. Werfen
7.3.4.8.1. Company overview
7.3.4.8.2. Financial performance
7.3.4.8.3. Product benchmarking
7.3.4.8.4. Strategic initiatives
7.3.4.9. Nova Biomedical
7.3.4.9.1. Company overview
7.3.4.9.2. Financial performance
7.3.4.9.3. Product benchmarking
7.3.4.9.4. Strategic initiatives
7.3.4.10. Trividia Health, Inc.
7.3.4.10.1. Company overview
7.3.4.10.2. Financial performance
7.3.4.10.3. Product benchmarking
7.3.4.10.4. Strategic initiatives
7.3.4.11. QuidelOrtho Corporation
7.3.4.11.1. Company overview
7.3.4.11.2. Financial performance
7.3.4.11.3. Product benchmarking
7.3.4.11.4. Strategic initiatives
7.3.4.12. Trinity Biotech
7.3.4.12.1. Company overview
7.3.4.12.2. Financial performance
7.3.4.12.3. Product benchmarking
7.3.4.12.4. Strategic initiatives
7.3.4.13. Sekisui Diagnostics
7.3.4.13.1. Company overview
7.3.4.13.2. Financial performance
7.3.4.13.3. Product benchmarking
7.3.4.13.4. Strategic initiatives
7.3.4.14. Orasure Technologies, Inc.
7.3.4.14.1. Company overview
7.3.4.14.2. Financial performance
7.3.4.14.3. Product benchmarking
7.3.4.14.4. Strategic initiatives
7.3.4.15. Spectral Medical, Inc.
7.3.4.15.1. Company overview
7.3.4.15.2. Financial performance
7.3.4.15.3. Product benchmarking
7.3.4.15.4. Strategic initiatives
7.3.4.16. EKF Diagnostics Holdings plc.
7.3.4.16.1. Company overview
7.3.4.16.2. Financial performance
7.3.4.16.3. Product benchmarking
7.3.4.16.4. Strategic initiatives
7.3.4.17. Anbio Biotechnology Co., Ltd.
7.3.4.17.1. Company overview
7.3.4.17.2. Financial performance
7.3.4.17.3. Product benchmarking
7.3.4.17.4. Strategic initiatives
7.3.4.18. AccuBioTech Co., Ltd
7.3.4.18.1. Company overview
7.3.4.18.2. Financial performance
7.3.4.18.3. Product benchmarking
7.3.4.18.4. Strategic initiatives
7.3.4.19. ALPHA LABORATORIES
7.3.4.19.1. Company overview
7.3.4.19.2. Financial performance
7.3.4.19.3. Product benchmarking
7.3.4.19.4. Strategic initiatives
7.3.5. Company Market Positioning Analysis, 2023
7.3.5.1. Glucose Monitoring
7.3.5.2. Cardiac Markers
7.3.5.3. Hematology
7.3.5.4. Coagulation
7.3.5.5. Fertility Testing
7.3.5.6. Infectious Diseases
7.3.5.7. Decentralized Clinical Chemistry
7.3.5.8. Oncology Markers
7.3.5.9. Blood Gas Testing
7.3.5.10. Ambulatory Testing
7.3.5.11. Drug of Abuse
7.3.5.12. Urinalysis
7.3.6. Key Parameters
7.3.6.1. Company Size
7.3.6.2. Geographic Presence
7.3.6.3. Production Portfolio
7.3.6.4. Collaborations
7.3.7. Market Differentiators
7.3.7.1. Application Trends
7.3.7.2. Technology Trends
7.3.7.3. Test Location Trends
7.3.7.4. End-use Trends
7.3.7.5. Regional Trends
7.3.8. Private Companies
7.3.8.1. New Entrants/Emerging Players
7.3.9. Regional Mapping: Public and Private Companies
Chapter 8. Conclusion
List of Tables
Table 1 List of abbreviation
Table 2 North America point of care diagnostics market, by region, 2018 - 2030 (USD Billion)
Table 3 North America point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 4 North America point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 5 U.S. point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 6 U.S. point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 7 Canada point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 8 Canada point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 9 Europe point of care diagnostics market, by region, 2018 - 2030 (USD Billion)
Table 10 Europe point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 11 Europe point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 12 Germany point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 13 Germany point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 14 UK point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 15 UK point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 16 France point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 17 France point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 18 Italy point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 19 Italy point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 20 Spain point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 21 Spain point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 22 Russia point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 23 Russia point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 24 Denmark point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 25 Denmark point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 26 Sweden point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 27 Sweden point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 28 Norway point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 29 Norway point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 30 Asia Pacific point of care diagnostics market, by region, 2018 - 2030 (USD Billion)
Table 31 Asia Pacific point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 32 Asia Pacific point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 33 China point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 34 China point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 35 Japan point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 36 Japan point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 37 India point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 38 India point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 39 South Korea point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 40 South Korea point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 41 Australia point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 42 Australia point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 43 Thailand point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 44 Thailand point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 45 Latin America point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 46 Latin America point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 47 Brazil point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 48 Brazil point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 49 Mexico point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 50 Mexico point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 51 Argentina point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 52 Argentina point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 53 MEA point of care diagnostics market, by region, 2018 - 2030 (USD Billion)
Table 54 MEA point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 55 MEA point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 56 South Africa point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 57 South Africa point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 58 Saudi Arabia point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 59 Saudi Arabia point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 60 UAE point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 61 UAE point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
Table 62 Kuwait point of care diagnostics market, by product, 2018 - 2030 (USD Billion)
Table 63 Kuwait point of care diagnostics market, by end use, 2018 - 2030 (USD Billion)
List of Figures
Fig. 1 Market research process
Fig. 2 Data triangulation techniques
Fig. 3 Primary research pattern
Fig. 4 Primary interviews in North America
Fig. 5 Primary interviews in Europe
Fig. 6 Primary interviews in APAC
Fig. 7 Primary interviews in Latin America
Fig. 8 Primary interviews in MEA
Fig. 9 Market research approaches
Fig. 10 Value-chain-based sizing & forecasting
Fig. 11 QFD modeling for market share assessment
Fig. 12 Market formulation & validation
Fig. 13 Point of care diagnostics market: market outlook
Fig. 14 Point of care diagnostics competitive insights
Fig. 15 Parent market outlook
Fig. 16 Related/ancillary market outlook
Fig. 17 Penetration and growth prospect mapping
Fig. 18 Industry value chain analysis
Fig. 19 Point of care diagnostics market driver impact
Fig. 20 Point of care diagnostics market restraint impact
Fig. 21 Point of care diagnostics market strategic initiatives analysis
Fig. 22 Point of care diagnostics market: Product movement analysis
Fig. 23 Point of care diagnostics market: Product outlook and key takeaways
Fig. 24 Glucose testing market estimates and forecast, 2018 - 2030
Fig. 25 Hb1Ac testing market estimates and forecast, 2018 - 2030
Fig. 26 Coagulation testing market estimates and forecast, 2018 - 2030
Fig. 27 Fertility/pregnancy market estimates and forecast, 2018 - 2030
Fig. 28 Infectious disease market estimates and forecast, 2018 - 2030
Fig. 29 HIV POC market estimates and forecast, 2018 - 2030
Fig. 30 Pneumonia or streptococcus associated infections market estimates and forecasts, 2018 - 2030
Fig. 31 Respiratory syncytial virus (RSV) POC market estimates and forecasts, 2018 - 2030
Fig. 32 HPV POC market estimates and forecasts, 2018 - 2030
Fig. 33 Influenza/ flu POC market estimates and forecasts, 2018 - 2030
Fig. 34 HCV POC market estimates and forecasts, 2018 - 2030
Fig. 35 MRSA POC market estimates and forecasts, 2018 - 2030
Fig. 36 TB and drug resistant TB POC market estimates and forecasts, 2018 - 2030
Fig. 37 HSV POC market estimates and forecasts, 2018 - 2030
Fig. 38 COVID-19 market estimates and forecasts, 2018 - 2030
Fig. 39 Other infectious diseases estimates and forecasts, 2018 - 2030
Fig. 40 Cardiac markers market estimates and forecasts, 2018 - 2030
Fig. 41 Thyroid stimulating hormone market estimates and forecasts, 2018 - 2030
Fig. 42 Hematology market estimates and forecasts, 2018 - 2030
Fig. 43 Primary care systems estimates and forecasts, 2018 - 2030
Fig. 44 Decentralized clinical chemistry market estimates and forecasts, 2018 - 2030
Fig. 45 Feces market estimates and forecasts, 2018 - 2030
Fig. 46 Lipid testing market estimates and forecasts, 2018 - 2030
Fig. 47 Cancer marker estimates and forecasts, 2018 - 2030
Fig. 48 Blood gas/ electrolytes market estimates and forecasts, 2018 - 2030
Fig. 49 Ambulatory chemistry market estimates and forecasts, 2018 - 2030
Fig. 50 Drug of abuse (DoA) testing market estimates and forecasts, 2018 - 2030
Fig. 51 Autoimmune diseases market estimates and forecasts, 2018 - 2030
Fig. 52 Urinalysis/nephrology market estimates and forecasts, 2018 - 2030
Fig. 53 Point of care diagnostics market: end use movement analysis
Fig. 54 Point of care diagnostics market: end use outlook and key takeaways
Fig. 55 Clinics market estimates and forecasts, 2018 - 2030
Fig. 56 Pharmacy & retail clinics market estimates and forecasts, 2018 - 2030
Fig. 57 Physician office market estimates and forecasts, 2018 - 2030
Fig. 58 Urgent care clinics market estimates and forecasts, 2018 - 2030
Fig. 59 Non-practice clinics market estimates and forecasts, 2018 - 2030
Fig. 60 Hospitals market estimates and forecasts, 2018 - 2030
Fig. 61 Home market estimates and forecasts, 2018 - 2030
Fig. 62 Assisted living healthcare facilities market estimates and forecasts, 2018 - 2030
Fig. 63 Laboratory market estimates and forecasts, 2018 - 2030
Fig. 64 Global point of care diagnostics market: Regional movement analysis
Fig. 65 Global point of care diagnostics market: Regional outlook and key takeaways
Fig. 66 Global point of care diagnostics market share and leading players
Fig. 67 North America market share and leading players
Fig. 68 Europe market share and leading players
Fig. 69 Asia Pacific market share and leading players
Fig. 70 Latin America market share and leading players
Fig. 71 Middle East & Africa market share and leading players
Fig. 72 North America: SWOT
Fig. 73 Europe SWOT
Fig. 74 Asia Pacific SWOT
Fig. 75 Latin America SWOT
Fig. 76 MEA SWOT
Fig. 77 North America, by country
Fig. 78 North America
Fig. 79 North America market estimates and forecasts, 2018 - 2030
Fig. 80 U.S. key country dynamics
Fig. 81 U.S. market estimates and forecasts, 2018 - 2030
Fig. 82 Canada country dynamics
Fig. 83 Canada market estimates and forecasts, 2018 - 2030
Fig. 84 Europe
Fig. 85 Europe market estimates and forecasts, 2018 - 2030
Fig. 86 UK country dynamics
Fig. 87 UK market estimates and forecasts, 2018 - 2030
Fig. 88 Germany country dynamics
Fig. 89 Germany market estimates and forecasts, 2018 - 2030
Fig. 90 France country dynamics
Fig. 91 France market estimates and forecasts, 2018 - 2030
Fig. 92 Italy country dynamics
Fig. 93 Italy market estimates and forecasts, 2018 - 2030
Fig. 94 Spain country dynamics
Fig. 95 Spain market estimates and forecasts, 2018 - 2030
Fig. 96 Russia country dynamics
Fig. 97 Russia market estimates and forecasts, 2018 - 2030
Fig. 98 Denmark country dynamics
Fig. 99 Denmark market estimates and forecasts, 2018 - 2030
Fig. 100 Sweden country dynamics
Fig. 101 Sweden market estimates and forecasts, 2018 - 2030
Fig. 102 Norway country dynamics
Fig. 103 Norway market estimates and forecasts, 2018 - 2030
Fig. 104 Asia Pacific
Fig. 105 Asia Pacific market estimates and forecasts, 2018 - 2030
Fig. 106 China country dynamics
Fig. 107 China market estimates and forecasts, 2018 - 2030
Fig. 108 Japan country dynamics
Fig. 109 Japan market estimates and forecasts, 2018 - 2030
Fig. 110 India country dynamics
Fig. 111 India market estimates and forecasts, 2018 - 2030
Fig. 112 Thailand country dynamics
Fig. 113 Thailand market estimates and forecasts, 2018 - 2030
Fig. 114 South Korea country dynamics
Fig. 115 South Korea market estimates and forecasts, 2018 - 2030
Fig. 116 Australia country dynamics
Fig. 117 Australia market estimates and forecasts, 2018 - 2030
Fig. 118 Latin America
Fig. 119 Latin America market estimates and forecasts, 2018 - 2030
Fig. 120 Brazil country dynamics
Fig. 121 Brazil market estimates and forecasts, 2018 - 2030
Fig. 122 Mexico country dynamics
Fig. 123 Mexico market estimates and forecasts, 2018 - 2030
Fig. 124 Argentina country dynamics
Fig. 125 Argentina market estimates and forecasts, 2018 - 2030
Fig. 126 Middle East and Africa
Fig. 127 Middle East and Africa market estimates and forecasts, 2018 - 2030
Fig. 128 South Africa country dynamics
Fig. 129 South Africa market estimates and forecasts, 2018 - 2030
Fig. 130 Saudi Arabia country dynamics
Fig. 131 Saudi Arabia market estimates and forecasts, 2018 - 2030
Fig. 132 UAE country dynamics
Fig. 133 UAE market estimates and forecasts, 2018 - 2030
Fig. 134 Kuwait country dynamics
Fig. 135 Kuwait market estimates and forecasts, 2018 - 2030
Fig. 136 Market share of key market players- Point of care diagnostics marketWhat questions do you have? Get quick response from our industry experts. Request a Free ConsultationMarket Segmentation
- Point Of Care Diagnostics Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Point Of Care Diagnostics End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Point Of Care Diagnostics Regional Outlook (Revenue, USD Billion, 2018 - 2030)
- North America
- North America Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- North America End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- U.S.
- U.S. Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- U.S. End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- U.S. Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Canada
- Canada Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Canada End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Canada Product Outlook (Revenue in USD Billion, 2018 - 2030)
- North America Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Europe
- Europe Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Europe End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Germany
- Germany Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Germany End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Germany Product Outlook (Revenue in USD Billion, 2018 - 2030)
- U.K.
- U.K. Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- U.K. End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- U.K. Product Outlook (Revenue in USD Billion, 2018 - 2030)
- France
- France Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- France End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- France Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Italy
- Italy Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Italy End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Italy Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Spain
- Spain Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Spain End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Spain Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Russia
- Russia Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Russia End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Russia Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Denmark
- Denmark Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Denmark End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Denmark Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Sweden
- Sweden Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Sweden End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Sweden Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Norway
- Norway Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Norway End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Norway Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Europe Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Asia Pacific
- Asia Pacific Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Asia Pacific End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Japan
- Japan Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Japan End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Japan Product Outlook (Revenue in USD Billion, 2018 - 2030)
- China
- China Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- China End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- China Product Outlook (Revenue in USD Billion, 2018 - 2030)
- India
- India Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- India End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- India Product Outlook (Revenue in USD Billion, 2018 - 2030)
- South Korea
- South Korea Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- South Korea End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- South Korea Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Australia
- Australia Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Australia End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Australia Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Thailand
- Thailand Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Thailand End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Thailand Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Asia Pacific Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Latin America
- Latin America Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Latin America End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Brazil
- Brazil Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Brazil End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Brazil Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Mexico
- Mexico Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Mexico End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Mexico Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Argentina
- Argentina Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Argentina End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Argentina Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Latin America Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Middle East and Africa (MEA)
- MEA Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- MEA End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- South Africa
- South Africa Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- South Africa End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- South Africa Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Saudi Arabia
- Saudi Arabia Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Saudi Arabia End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- UAE
- UAE Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- UAE End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- UAE Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Kuwait
- Kuwait Product Outlook (Revenue in USD Billion, 2018 - 2030)
- Glucose Testing
- Hb1Ac Testing
- Coagulation Testing
- Fertility/Pregnancy
- Infectious Disease
- HIV POC
- Clostridium Difficile POC
- HBV POC
- Pneumonia or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and Drug-resistant TB POC
- HSV POC
- COVID-19
- Other Infectious Diseases
- Cardiac Markers
- Thyroid Stimulating Hormone
- Hematology
- Primary Care Systems
- Decentralized Clinical Chemistry
- Feces
- Lipid Testing
- Cancer Marker
- Blood Gas/Electrolytes
- Ambulatory Chemistry
- Drug of Abuse (DOA) Testing
- Autoimmune Diseases
- Urinalysis/Nephrology
- Kuwait End Use Outlook (Revenue in USD Billion, 2018 - 2030)
- Clinics
- Pharmacy & Retail Clinics
- Physician Office
- Urgent Care Clinics
- Non-practice Clinics
- Hospitals
- Home
- Assisted Living Healthcare Facilities
- Laboratory
- Clinics
- Kuwait Product Outlook (Revenue in USD Billion, 2018 - 2030)
- MEA Product Outlook (Revenue in USD Billion, 2018 - 2030)
- North America
Point Of Care Diagnostics Market Dynamics
Driver: Growing Geriatric Population Base
The increasing geriatric population is also contributing to the expansion of the point-of-care diagnostics market, as the elderly population is more susceptible to chronic diseases, including diabetes, cardiovascular disorders, & respiratory illnesses. Point-of-care diagnostics play a vital role in managing these conditions by enabling convenient and regular monitoring of biomarkers, such as blood glucose levels, cholesterol, & lung function. As the global geriatric population continues to grow, the demand for point-of-care diagnostic devices and tests is expected to rise, driving market growth.
The growing geriatric population seeking companionship, security, and help with daily activities is a major element driving segment growth. Increased life expectancy is expected to result in rapid growth of the geriatric population in the coming years. According to the National Institute on Aging (NIA), about 8.5% of the world's population is aged 65 and above. According to the Population Reference Bureau, people aged 65 and above in the U.S. are expected to increase from 55.8 million in 2020 to 95 million by 2060. In addition, the global market for ALFs is likely to be driven by technological advancements. The market is expected to benefit from the development of advanced and easy-to-use equipment and services, including Internet-enabled home monitoring, telemedicine, and mobile health apps over the projected period
Driver: Growing Prevalence of Target Diseases
The high prevalence of cardiac diseases and the need for effective & rapid diagnostic procedures (POC) in an attempt to timely curb the incidence rates are expected to support the projected growth. According to statistics published by the International Diabetes Federation, the global prevalence of diabetes will increase from 537 million in 2021 to 643 million by 2030 and is expected to reach 783 million by 2045. Moreover, increasing demand for home healthcare due to the rising prevalence of diabetes is anticipated to drive market growth. Owing to the growing demand for home healthcare-based POC diagnostic procedures in the rural and remote areas of countries such as India & China, the need for OTC and rapid diagnostic kits is expected to increase over the next 7 years, causing a rise in revenue generation.
Regular glucose testing is of utmost importance for diabetic patients. As per Diabetes Control and Complications Trial, the use of blood glucose monitors helps in lowering disease complications. POC glucose monitors are used for periodic monitoring of blood glucose, which helps doctors decide on appropriate treatment patterns. Over-the-counter (OTC) or rapid tests and prescription tests are the POC tests that are useful for the determination of blood glucose levels in hospitals and other POC settings. The rising prevalence of diabetes and the introduction of technologically advanced and portable diagnostic solutions are expected to boost market growth during the forecast period. For instance, in January 2022, Roche announced the launch of a hand-held, smart glucose meter to be used in hospital settings. Blood glucose POC diagnostics have gained immense demand, which can be attributed to convenient handling and associated affordability. This enables the near-to-immediate assessment of patients who are critically ill, wherein immediate test results are imperative to design further treatment approaches. Thus, glucose POC diagnostic solutions are also becoming a valuable resource in the U.S., with more than 20 million residents affected by diabetes, propelling the concept of self-testing and on-site testing.
Restraint: Presence of Ambiguous Regulatory and Reimbursement Framework for Primary Care Setting
Healthcare professionals operating across hospital settings face several issues, such as complex regulatory demand for accreditation of POC, satisfaction levels of the personnel, lack of coordination between clinical groups, and its impact on the alternative tests introduced in the market. The U.S. FDA and the Center for Devices and Radiological Health are the regulatory agencies that approve POC tests through the 510(K) process. ISO 15189 indicates requirements for competence and quality in medical laboratories. It can be used by medical laboratories to develop their quality management systems to assess their own expertise in POCT. It can also be used for recognizing or confirming the competency of medical laboratories by regulating authorities, accreditation bodies, and laboratory customers. Federal regulation of POCT is minimal, and accrediting agencies and states regularly enforce additional requirements. It focuses on two areas:
-
Competency and training of the person conducting the testing
-
Authentication of stringent adherence to the manufacturer-specified method for every test.
Most plans rely on the CMS fee schedule as a reference point; the internal review may also be conducted by a health plan. Payers would not reimburse providers for the cost of any additional instrumentation and equipment of the test reader. These costs have to be recovered in the fee schedule for claims to perform the test. The reimbursement policies are complex and regionally discrepant. For instance, in China, there is no established protocol for being included in the list or qualifying for reimbursement. In China, the National Drug Reimbursement List (NDRL) must align with the expenses that can be covered by the Basic Medical Insurance (BMI) funds on an annual basis.
What Does This Report Include?
This section will provide insights into the contents included in this point of care diagnostics market report and help gain clarity on the structure of the report to assist readers in navigating smoothly.
Point of care diagnostics market qualitative analysis
-
Industry overview
-
Industry trends
-
Market drivers and restraints
-
Market size
-
Growth prospects
-
Porter’s analysis
-
PESTEL analysis
-
Key market opportunities prioritized
-
Competitive landscape
-
Company overview
-
Financial performance
-
Product benchmarking
-
Latest strategic developments
-
Point of care diagnostics market quantitative analysis
-
Market size, estimates, and forecast from 2018 to 2030
-
Market estimates and forecast for product segments up to 2030
-
Regional market size and forecast for product segments up to 2030
-
Market estimates and forecast for application segments up to 2030
-
Regional market size and forecast for application segments up to 2030
-
Company financial performance
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationResearch Methodology
A three-pronged approach was followed for deducing the point of care diagnostics market estimates and forecasts. The process has three steps: information procurement, analysis, and validation. The whole process is cyclical, and steps repeat until the estimates are validated. The three steps are explained in detail below:
Information procurement: Information procurement is one of the most extensive and important stages in our research process, and quality data is critical for accurate analysis. We followed a multi-channel data collection process for point of care diagnostics market to gather the most reliable and current information possible.
- We buy access to paid databases such as Hoover’s and Factiva for company financials, industry information, white papers, industry journals, SME journals, and more.
- We tap into Grand View’s proprietary database of data points and insights from active and archived monitoring and reporting.
- We conduct primary research with industry experts through questionnaires and one-on-one phone interviews.
- We pull from reliable secondary sources such as white papers and government statistics, published by organizations like WHO, NGOs, World Bank, etc., Key Opinion Leaders (KoL) publications, company filings, investor documents, and more.
- We purchase and review investor analyst reports, broker reports, academic commentary, government quotes, and wealth management publications for insightful third-party perspectives.
Analysis: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilized different methods of point of care diagnostics market data depending on the type of information we’re trying to uncover in our research.
-
Market Research Efforts: Bottom-up Approach for estimating and forecasting demand size and opportunity, top-down Approach for new product forecasting and penetration, and combined approach of both Bottom-up and Top-down for full coverage analysis.
-
Value-Chain-Based Sizing & Forecasting: Supply-side estimates for understanding potential revenue through competitive benchmarking, forecasting, and penetration modeling.
-
Demand-side estimates for identifying parent and ancillary markets, segment modeling, and heuristic forecasting.
-
Qualitative Functional Deployment (QFD) Modelling for market share assessment.
Market formulation and validation: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilize different methods of data analysis depending on the type of information we’re trying to uncover in our research.
-
Market Formulation: This step involves the finalization of market numbers. This step on an internal level is designed to manage outputs from the Data Analysis step.
-
Data Normalization: The final market estimates and forecasts are then aligned and sent to industry experts, in-panel quality control managers for validation.
-
This step also entails the finalization of the report scope and data representation pattern.
-
Validation: The process entails multiple levels of validation. All these steps run in parallel, and the study is forwarded for publishing only if all three levels render validated results.
Point Of Care Diagnostics Market Categorization:
The point of care diagnostics market was categorized into three segments, namely product (Glucose Testing, Hb1Ac Testing, Coagulation Testing, Fertility/Pregnancy, Infectious Disease, Cardiac Markers, Thyroid Stimulating Hormone, Hematology, Primary Care Systems, Decentralized Clinical Chemistry, Feces, Lipid Testing, Cancer Marker, Blood Gas/Electrolytes, Ambulatory Chemistry, Drug of Abuse (DOA) Testing, Urinalysis/Nephrology), end-use (Clinics, Hospitals, Home, Assisted Living Healthcare Facilities, Laboratory), regions (North America, Europe, Asia Pacific, Latin America, Middle East & Africa).
Segment Market Methodology:
The point of care diagnostics market was segmented into product, end-use, and regions. The demand at a segment level was deduced using a funnel method. Concepts like the TAM, SAM, SOM, etc., were put into practice to understand the demand. We at GVR deploy three methods to deduce market estimates and determine forecasts. These methods are explained below:
Market research approaches: Bottom-up
-
Demand estimation of each product across countries/regions summed up to from the total market.
-
Variable analysis for demand forecast.
-
Demand estimation via analyzing paid database, and company financials either via annual reports or paid database.
-
Primary interviews for data revalidation and insight collection.
Market research approaches: Top-down
-
Used extensively for new product forecasting or analyzing penetration levels.
-
Tool used invoice product flow and penetration models Use of regression multi-variant analysis for forecasting Involves extensive use of paid and public databases.
-
Primary interviews and vendor-based primary research for variable impact analysis.
Market research approaches: Combined
- This is the most common method. We apply concepts from both the top-down and bottom-up approaches to arrive at a viable conclusion.
Regional Market Methodology:
The point of care diagnostics market was analyzed at a regional level. The globe was divided into North America, Europe, Asia Pacific, Latin America, Middle East & Africa, keeping in focus variables like consumption patterns, export-import regulations, consumer expectations, etc. These regions were further divided into seventeen countries, namely, the U.S.; Canada; Germany; the UK; France; Italy; Spain; Russia; China; Japan; India; Australia; South Korea; Brazil; Mexico; South Africa; Saudi Arabia.
All three above-mentioned market research methodologies were applied to arrive at regional-level conclusions. The regions were then summed up to form the global market.
Point of care diagnostics market companies & financials:
The point of care diagnostics market was analyzed via companies operating in the sector. Analyzing these companies and cross-referencing them to the demand equation helped us validate our assumptions and conclusions. Key market players analyzed include:
-
F. Hoffman-La Roche Ltd.: F. Hoffmann-La Roche Ltd. develops and manufactures diagnostic & pharmaceutical products. It has 26 manufacturing sites globally with two business segments: pharmaceuticals and diagnostics. The pharmaceutical segment manufactures drugs for infectious diseases, cardiovascular, respiratory, & metabolic disorders, cancer, and central nervous system & autoimmune diseases. Roche Diagnostics is involved in various fields ranging from patient self-monitoring to clinical laboratory systems. Roche Professional Diagnostics (RPD) is the company’s largest business segment and provides solutions, tests, and instrument systems to enable reliable and cost-effective delivery of results. Ventana Medical Systems, a member of Roche Group, is involved in developing assays & kits for in situ hybridization. It has operations in several regions, including North America, South America, Europe, Africa, Asia, and Oceania.
-
QIAGEN: QIAGEN is a biotechnology company operating in over 20 countries globally. Key business areas of the company include assay technologies for molecular diagnostics, pharmaceutical, academic, and applied testing. Furthermore, molecular technologies developed by the company are being utilized by forensic technicians, and the company is also involved in the isolation of genetic material from biological samples.
-
Danaher Corporation: Danaher designs, manufactures, and commercializes products as well as services catering to medical, professional, and commercial applications. The company operates through four segments: diagnostics, life sciences, environmental & applied solutions, and dental solutions. Danaher’s diagnostics segment offers reagents, analytical instruments, software, consumables, and services for physicians’ offices, hospitals, reference laboratories, and other critical care settings to diagnose various diseases.
-
Becton Dickinson: Becton, Dickinson, and Company is a publicly held medical technology company. The company operates through three business units, namely BD Medical, BD International, and BD Life Sciences. The company offers innovative technology solutions and services for clinical therapy. Through the BD Life Sciences segment, the company offers preanalytical systems, diagnostic systems, and bioscience solutions. The company is majorly engaged in the development, manufacture, and sales of instrument systems, medical devices, and reagents required in clinical laboratories, life sciences researchers, healthcare institutions, and the pharmaceutical industry.
-
bioMérieux: bioMérieux is a multinational biotechnology company that specializes in the field of IVD for medical and industrial sectors. The company designs, develops, and produces diagnostic systems and provides diagnostic solutions such as reagents, instruments, and software. The company’s products are widely used for diagnosing infectious diseases and testing for cardiovascular disorders as well as cancer screening and monitoring. Currently, it has operations in more than 150 countries through its distribution network and 42 subsidiaries.
-
Abbott: Abbott is a healthcare company with five core business segments: pharmaceuticals, medical devices, nutrition, vascular care, and diagnostics. The company is present in more than 150 countries. In October 2011, Abbott Laboratories announced its decision to separate into two companies—one focused on research-based pharmaceuticals and the other on diversified medical products. In September 2017, Abbott completed the acquisition of Alere, Inc. to expand its diagnostics business.
-
Siemens Healthcare GmbH: Siemens Healthcare GmbH operates as a subsidiary of Siemens Group of Companies. Its business is segmented into laboratory diagnostics, diagnostic imaging, POC diagnostics, ultrasound, advanced therapies, and services. Its R&D activities in healthcare are focused on developing therapies, molecular diagnostics, and genetic services. It is focused on utilizing the potential of imaging solutions in therapy to bridge the gap between diagnostics and therapy in interventional clinical disciplines, cardiology, radiation oncology, and surgery. Its product & solution offerings include POC testing, healthcare IT, medical imaging, services, laboratory diagnostics, and clinical specialties & diseases. The company is present across Europe, Africa, the Middle East, North America, Asia, and Australia.
-
Werfen: Instrumentation Laboratory (IL) is a part of the Werfen Group, a healthcare corporation dealing in IVD products, scientific instruments, and medical devices. The company provides diagnostic instruments for hemostasis and critical care. It focuses strongly on R&D and holds over 100 patents about the advancement of medicine. The company has a significant geographical presence across North America, EMEA, and Asia Pacific.
-
Nova Biomedical: Nova Biomedical is a privately owned company involved in the development, manufacturing, and sale of advanced technology blood testing analyzers that employ optical measuring and electrochemical techniques. It is one of the most prominent in vitro diagnostics companies worldwide. The company’s sales & service subsidiaries are present in seven countries, and distributors are in over 92 countries worldwide. Nova Biomedical’s manufacturing facilities are based in Massachusetts and Taiwan.
-
Trividia Health, Inc.: Trividia Health, Inc. is a nonpublic company that was acquired by Sinocare, Inc. in January 2016. Sinocare, Inc. specializes in the development and distribution of rapid diagnostic testing in China. In addition, the company also manufactures several blood glucose monitoring systems including Safe-Accu, Safe-AQ, Gold-Accu, and Safe-Accu UG, and D Nurse mobile glucose meters. Trividia Health is a medical devices company that supplies co-branded blood glucose monitoring systems to retailers throughout North America. The company’s product portfolio consists of blood glucose meters & test strips, sharps, skincare, and other diabetes management tools. Currently, the company has manufacturing facilities in Florida, Taiwan, and New Hampshire.
Value chain-based sizing & forecasting
Supply Side Estimates
-
Company revenue estimation via referring to annual reports, investor presentations, and Hoover’s.
-
Segment revenue determination via variable analysis and penetration modeling.
-
Competitive benchmarking to identify market leaders and their collective revenue shares.
-
Forecasting via analyzing commercialization rates, pipelines, market initiatives, distribution networks, etc.
Demand side estimates
-
Identifying parent markets and ancillary markets
-
Segment penetration analysis to obtain pertinent
-
revenue/volume
-
Heuristic forecasting with the help of subject matter experts
-
Forecasting via variable analysis
Point Of Care Diagnostics Market Report Objectives:
-
Understanding market dynamics (in terms of drivers, restraints, & opportunities) in the countries.
-
Understanding trends & variables in the individual countries & their impact on growth and using analytical tools to provide high-level insights into the market dynamics and the associated growth pattern.
-
Understanding market estimates and forecasts (with the base year as 2022, historic information from 2019 to 2021, and forecast from 2023 to 2030). Regional estimates & forecasts for each category are available and are summed up to form the global market estimates.
Point Of Care Diagnostics Market Report Assumptions:
-
The report provides market value for the base year 2022 and a yearly forecast till 2030 in terms of revenue/volume or both. The market for each of the segment outlooks has been provided on region & country basis for the above-mentioned forecast period.
-
The key industry dynamics, major technological trends, and application markets are evaluated to understand their impact on the demand for the forecast period. The growth rates were estimated using correlation, regression, and time-series analysis.
-
We have used the bottom-up approach for market sizing, analyzing key regional markets, dynamics, & trends for various products and end-users. The total market has been estimated by integrating the country markets.
-
All market estimates and forecasts have been validated through primary interviews with the key industry participants.
-
Inflation has not been accounted for to estimate and forecast the market.
-
Numbers may not add up due to rounding off.
-
Europe consists of EU-8, Central & Eastern Europe, along with the Commonwealth of Independent States (CIS).
-
Asia Pacific includes South Asia, East Asia, Southeast Asia, and Oceania (Australia & New Zealand).
-
Latin America includes Central American countries and the South American continent
-
Middle East includes Western Asia (as assigned by the UN Statistics Division) and the African continent.
Primary Research
GVR strives to procure the latest and unique information for reports directly from industry experts, which gives it a competitive edge. Quality is of utmost importance to us, therefore every year we focus on increasing our experts’ panel. Primary interviews are one of the critical steps in identifying recent market trends and scenarios. This process enables us to justify and validate our market estimates and forecasts to our clients. With more than 8,000 reports in our database, we have connected with some key opinion leaders across various domains, including healthcare, technology, consumer goods, and the chemical sector. Our process starts with identifying the right platform for a particular type of report, i.e., emails, LinkedIn, seminars, or telephonic conversation, as every report is unique and requires a differentiated approach.
We send out questionnaires to different experts from various regions/ countries, which is dependent on the following factors:
-
Report/Market scope: If the market study is global, we send questionnaires to industry experts across various regions, including North America, Europe, Asia Pacific, Latin America, and MEA.
-
Market Penetration: If the market is driven by technological advancements, population density, disease prevalence, or other factors, we identify experts and send out questionnaires based on region or country dominance.
The time to start receiving responses from industry experts varies based on how niche or well-penetrated the market is. Our reports include a detailed chapter on the KoL opinion section, which helps our clients understand the perspective of experts already in the market space.
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationShare this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





