- Home
- »
- Clinical Diagnostics
- »
-
Genetic Disease Diagnostics Market Size, Share Report 2033GVR Report cover
![Genetic Disease Diagnostics Market Size, Share & Trends Report]()
Genetic Disease Diagnostics Market (2025 - 2033) Size, Share & Trends Analysis Report By Technology (NGS, PCR-based Testing), By Channel (Online, Offline), By Application, By Product, By End Use, By Region, And Segment Forecasts
- Report ID: GVR-4-68040-814-0
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2021 - 2024
- Forecast Period: 2025 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
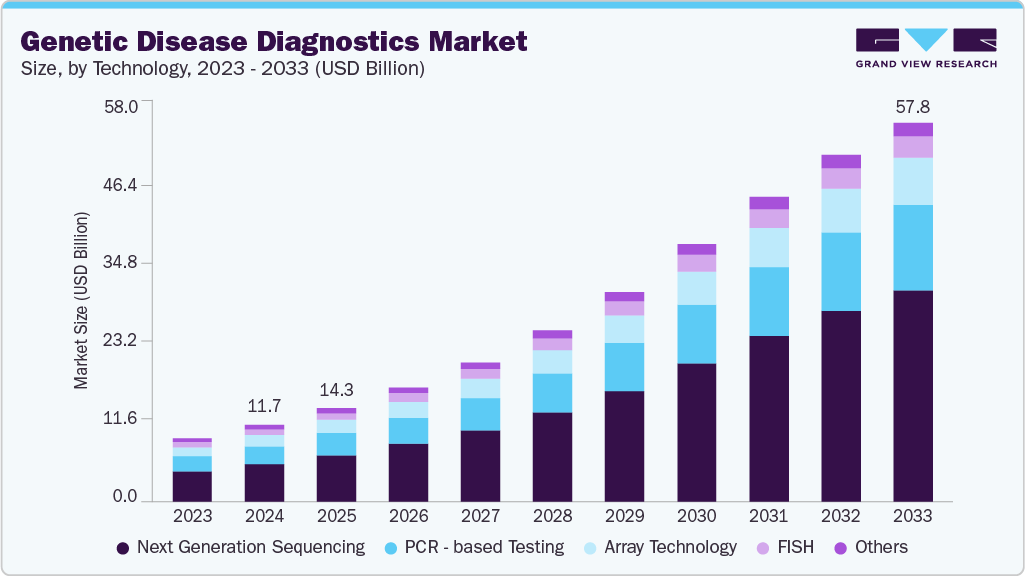
Genetic Disease Diagnostics Market Summary
The global genetic disease diagnostics market size was estimated at USD 11.71 billion in 2024 and is projected to reach USD 57.83 billion by 2033, growing at a CAGR of 19.1% from 2025 to 2033. The market is primarily driven by technological advancements, increasing demand for personalized medicine, and growing demand for newborn screening..
Key Market Trends & Insights
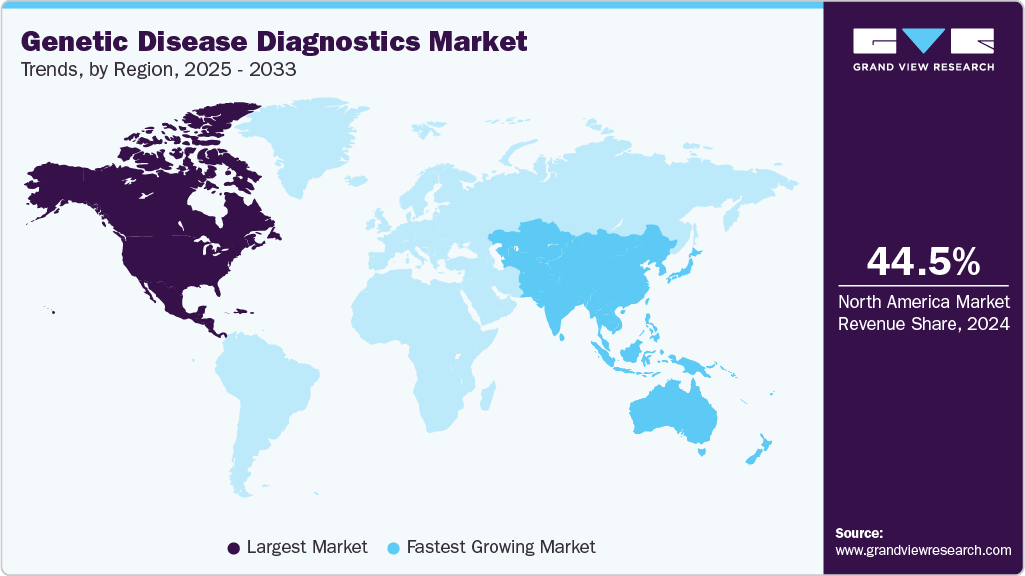
- North America genetic disease diagnostics market dominated the global market with the largest revenue share of 44.49% in 2024.
- The genetic disease diagnostics market in the U.S. is projected to grow significantly during the forecast period.
- Based on technology, the next-generation sequencing segment led the market with the largest revenue share of 49.3% in 2024.
- Based on application, the health and wellness-predisposition/risk/tendency segment led the market with the largest revenue share at 52.3% in 2024.
Market Size & Forecast
- 2024 Market Size: USD 11.71 Billion
- 2033 Projected Market Size: USD 57.83 Billion
- CAGR (2025-2033): 19.1%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
Genetic testing in maternity care is undergoing a major transformation, driven by advancements in carrier screening, non-invasive prenatal testing (NIPT), and IVF-related genetic analyses. These innovations are fueling consistent market growth, especially across both established and emerging economies.Pharmacogenomics testing is increasingly vital in helping clinicians choose the most effective medications and appropriate dosages for patients. The Association for Molecular Pathology (AMP) has made strides toward standardizing these tests across laboratories. In August 2022, the AMP released recommendations for designing and validating clinical genotyping assays for genes like NUDT15 and TPMT. These guidelines provide a minimum set of alleles for laboratories to identify patients who may be at a higher risk of thiopurine toxicity, thereby improving treatment safety and efficacy.
The introduction of new tests that can considerably reduce treatment costs and help lower pharmaceutical expenses by preventing overprescription and excessive dosing, while also reducing the risk of complications and hospitalizations resulting from adverse drug reactions, is expected to impact market growth positively. For instance, in October 2025, AIG Hospitals, a Hyderabad (India) based hospital, in collaboration with GenepowerRx, launched a pharmacogenomics test that would help doctors to prescribe medicines or decide on a treatment plan based on the patient's genetic makeup.
Driven primarily by advancements in Next-Generation Sequencing (NGS) technology, WGS costs are falling toward the sub-USD 100 threshold. Many companies have launched new tests based on next-generation sequencing (NGS), whole-genome sequencing, and advanced array technologies, offering higher accuracy, coverage, and rapid results. For instance, CENTOGENE launched CentoGenome Ultra-Fast, a whole-genome sequencing tool for diagnosing genetic diseases in five business days. Rapid diagnosis and early intervention facilitate timely treatment, which in turn improves patient outcomes and reduces complications in emergency cases and the ICU.
Despite these advancements, the high cost of the test may impede market growth in developing countries. Depending on the test's complexity, costs can range from under $100 to over $2,000, with higher costs associated with tests that require multiple family samples. Results may also take weeks to arrive. In some instances, health insurance covers these tests when prescribed by a medical professional, but coverage varies widely across insurers and policies.
Market Concentration & Characteristics
The genetic disease diagnostics industry is experiencing significant innovation, driven by advancements in liquid biopsy & non-invasive testing, leading to earlier diagnosis. Diagnostics moving from invasive sample types (e.g., tissue) to less invasive ones (blood, cfDNA), enabling earlier detection/monitoring of the disease, resulting in high adoption of testing.
The genetic disease diagnostics industry has witnessed robust mergers and acquisitions, as companies seek to consolidate capabilities, expand their portfolios, and drive growth through strategic acquisitions. For instance, Veritas Genetics acquired qGenomics in September 2025 and NIMGenetics in January 2025 to expand its rare disease and carrier screening portfolios. These instances demonstrate how M&A is being used strategically in the market to gain technology (AI, interpretation software), expand the test menu (rare diseases, carrier screening, hereditary cancer), and broaden the geographic reach.
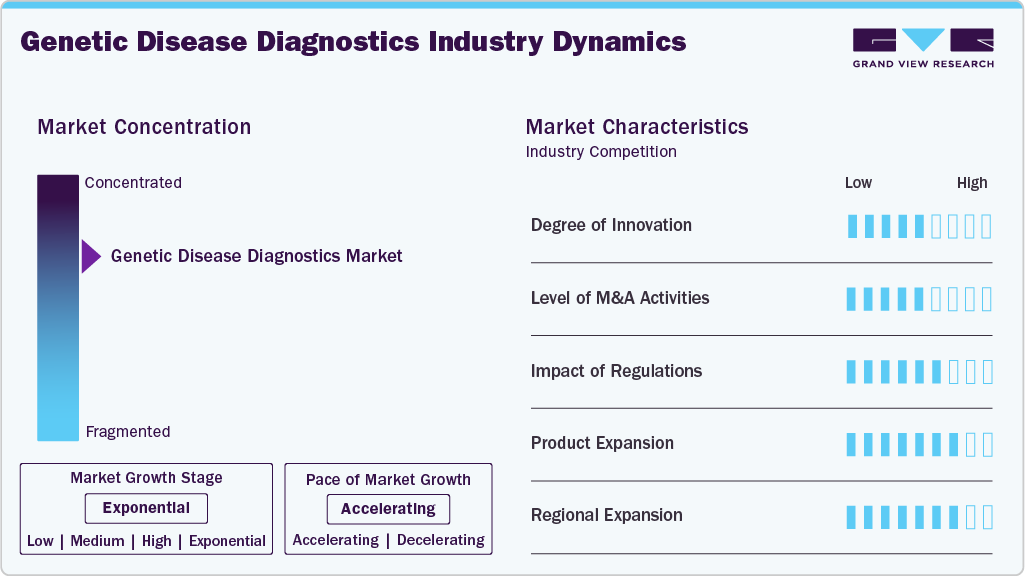
Regulatory frameworks have a significant influence on the genetic disease diagnostics industry. In the U.S., the FDA oversees in vitro diagnostic (IVD) products, ensuring safety and efficacy. In the EU, the transition from the previous In Vitro Diagnostics Directive (IVDD) to the new In Vitro Diagnostic Regulation (IVDR) significantly tightened requirements for manufacturers of tests or IVD devices. The new regulations also demand stronger post-market surveillance, lifecycle management of devices/tests, and risk-based classification of IVDs (especially genetic tests).
The market for genetic disease diagnostics is expanding with the development of new products that offer enhanced sensitivity and specificity. Innovations such as digital PCR (dPCR) and real-time quantitative PCR (qPCR) are enhancing analytical sensitivity and quantification accuracy. Companies are launching new multiplex PCR panels for the rapid detection of variants and SNPs relevant to inherited and oncology disorders. For instance, in May 2025, Guardant Health launched the Guardant Hereditary Cancer test, which covers ~82 genes and over 12 tumor types (including breast, colorectal, prostate, endometrial, and renal) via a blood-based sample.
The genetic disease diagnostics industry is experiencing regional expansion, particularly in the Asia-Pacific region. Regional expansion often involves local partnerships, distribution alliances, and localization of testing workflows (including language, regulatory, and population genetics), which facilitates faster adoption. For instance, in May 2023, MedGenome acquired Prognosis Laboratories, NCR’s one of the most reputed laboratories, to strengthen its diagnostics and expand regional penetration in India-based testing services.
Technology Insights
The Next Generation Sequencing segment accounted for 49.3% of the market in 2024 and is expected to grow at the fastest CAGR over the forecast period. The expansion of genetic disease diagnostics is driven by an increase in genome mapping initiatives, higher healthcare spending, advancements in technology, and the broader application of next-generation sequencing (NGS). DNA sequencing is increasingly utilized to identify and analyze various cancers and genetic disorders, with tumor DNA sequencing playing a critical role in detecting unique DNA changes. For instance, Tempus AI, a technology-based company, launched an NGS-based in vitro diagnostic device, xT CDx. The device is a 648-gene NGS test for solid tumor profiling, delivering insights with one of the largest reported gene panels.
PCR-based testing held the second-largest share in 2024, owing to the integration of advanced PCR technologies, growing clinical adoption, and an expanding role in reproductive and prenatal screening. Major players are launching next-gen PCR systems with high-throughput automated workflows. PCR assays are used for aneuploidy detection, carrier screening, and preimplantation genetic testing (PGT), enabling the precise selection of embryos in IVF and the early detection of risks. These applications align with the growing global emphasis on maternal and fetal health genomics.
Application Insights
The health and wellness-predisposition/risk/tendency segment captured the largest revenue share at 52.3% in 2024. This trend reflects the growing demand for predictive genetic disease diagnostics and consumer wellness genomics, driven by a heightened focus on healthy lifestyles and increased healthcare awareness. Rising interest in direct-to-consumer (DTC) genetic testing post-pandemic, as people seek preventive insights and personalized wellness strategies. 23andMe, AncestryDNA, and CircleDNA continue to expand into digital health platforms, integrating genetics with wearable or lifestyle data. For instance, in March 2024, Xcode Life introduced a new Genes and Caffeine” test. This test provides individualized insights into caffeine sensitivity and metabolism. This highlights the growing market for using genomic data for lifestyle optimization.
The genetic disease carrier status segment is also expected to grow significantly at a CAGR of 22.4% over the forecast period. Carrier testing evaluates ancestral DNA to identify potential genetic conditions, making it increasingly popular as the field of genetics advances. As a result, demand for carrier testing is likely to increase, further supporting overall genetic disease diagnostics industry growth. New product launches are likely to drive the market over the forecast period. In May 2025, CENTOGENE introduced a new Reproductive Genetics Portfolio, including PGT for Aneuploidy (PGT-A) and comprehensive carrier screening services (CentoScreen). PGT-A leverages CENTOGENE’s database of over one million sequences for high sensitivity and accuracy, with an optional rapid 24-hour turnaround for IVF embryos. CentoScreen covers ≥99% of 332 genes for individuals or couples, enabling early detection of inherited genetic risks.
Product Insights
The consumables segment captured the largest revenue share at 60.2% in 2024. The consumables market is poised for strong growth, driven by the consistent demand for reagents and accessories essential in testing processes. Many companies in this sector are pursuing both organic and inorganic strategies to expand their product lines and broaden their geographic reach. Moreover, as more assays become clinically adopted and reimbursed, labs increase testing with disposable reagents/kits, thereby supporting demand for consumables. For instance, in September 2023, Telesos Bio launched the BioXp NGS Library Prep kit for Whole Genome Sequencing on the BioXp 3250 and BioXp 9600 systems. This NGS library preparation kit allows automated, on-demand processing of up to 96 genomic DNA samples, streamlining library preparation for whole-genome sequencing applications.
The software & services segment is also expected to experience substantial growth of 24.8% during the forecast period, largely fueled by strategic investments from Contract Research Organizations (CROs) and integration of AI tools with genetic platforms. For instance, in February 2025, Fore Genomics partnered with Inocras to launch newborn screening and pediatric genetic health screening. Integration of Fore Genomics pediatric services with Inocras’s cutting-edge WGS & bioinformatics platform would enable healthcare providers & patients to gain precise, actionable, and personalized insights into the genomic health of children and newborns, further facilitating early diagnosis and proactive medical care. In addition, the increasing array of services, including those in skin and genetic testing, further supports growth in this segment.
Channel Insights
The offline segment captured the largest revenue share in 2024. The rapid development of molecular genetic disease diagnostics technologies has significantly expanded the genetic disease diagnostics industry. This progress has also spurred the growth of private diagnostic laboratories, which offer a diverse range of solutions. A notable trend in molecular-based testing services is the increasing availability of buccal swab collection kits and saliva-based tests, which can be purchased over the counter in pharmacies, providing consumers with convenient options. Offline facilities offer detailed analysis, genomic counseling, and in-person support, which are critical for complex or high-risk genetic tests. Many countries require medical supervision for certain genetic tests, favoring offline sample collection and testing.
The online channel is expected to exhibit the fastest market growth, with a projected CAGR of 23.6%. This growth is driven by increased research funding in molecular biology, the rising popularity of direct-to-consumer testing, and greater awareness and acceptance of personalized medicine. Direct-to-consumer companies utilize both online and over-the-counter (OTC) channels to provide accessible genetic disease diagnostic solutions. For instance, in February 2025, Targeted Genomics received FDA approval for the DTC GultenID test, the first at-home genetic test for celiac disease that can be available without a prescription. Furthermore, the continued strong demand for ancestry and relationship testing acts as a low-barrier entry point, introducing millions of users to the concept of genomic testing through an online channel.
End Use Insights
The hospitals and clinics segment captured the largest revenue share at 43.9% in 2024. The genetic disease diagnostics industry is being propelled by mandatory newborn screening for hereditary disorders and a growing prevalence of cancer across various regions. In the U.S., approximately 98% of births occur in hospitals, and an increase in hospital and clinic capacities is expected to drive demand for testing further. Supporting this growth, the Australian government announced a healthcare budget of USD 107.3 billion from 2022-23 to 2027-28, dedicated specifically to expanding newborn screening programs.
The adoption of telehealth and in-hospital services is also fueling market expansion. For instance, in 2024, there were over 116 million telehealth consultations worldwide, up from around 57 million in 2019. Telehealth enables a more efficient use of specialist time (genetic counsellors, clinical geneticists), which helps scale up testing services without necessarily scaling physical infrastructure. The integration of telehealth with at-home sample collection or remote test-ordering pathways expands the market reach for genetic testing.
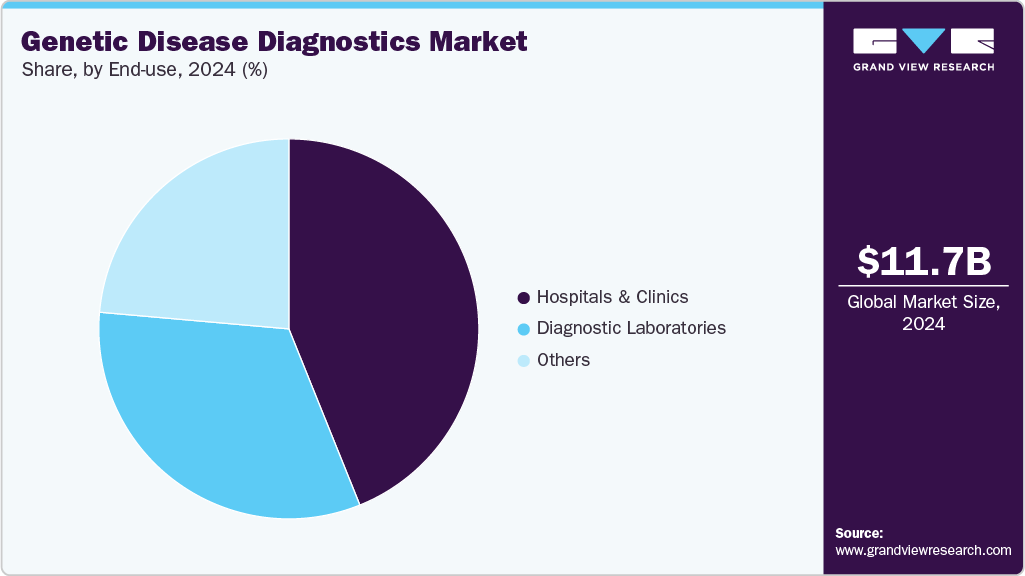
The diagnostic laboratories segment is expected to grow at a CAGR of 22.7%, driven by increased partnerships and collaborations with Genetic Disease Diagnostics companies. For instance, Thermo Fisher Scientific supports laboratories in conducting genomic research by providing advanced technologies such as next-generation sequencing (NGS) and quantitative PCR. These collaborations enable diagnostic labs to enhance their testing capabilities and expand their service offerings, contributing to the overall market growth. Diagnostic labs are increasingly bundling genetic testing with other diagnostics (e.g., oncology panels, prenatal screening, inherited disease panels). This cross-integration increases lab business and the share of the genetic testing workload.
Regional Insights
The North America genetic disease diagnostics market dominated and accounted for a 44.49% share in 2024. The rising demand for diagnostic services is largely driven by the growing prevalence of chronic illnesses and the advancement of diagnostic technologies. In addition, the increasing cases of infectious diseases, such as tuberculosis, HIV, and influenza, are expected to heighten the need for effective detection and treatment options. This demand is further supported by the region's well-established healthcare infrastructure and government funding for research, which are expected to collectively bolster market growth.
U.S. Genetic Disease Diagnostics Market Trends
The genetic disease diagnostics market in the U.S. is projected to grow significantly during the forecast period, driven by a combination of factors, including increasing awareness of the benefits of early disease detection, advancements in molecular biology, and a growing emphasis on personalized medicine.
Europe Genetic Disease Diagnostics Market Trends
The genetic disease diagnostics market in Europe is likely to emerge as a lucrative region in the industry. Advances in Genetic Disease Diagnostics technologies, such as next-generation sequencing (NGS), have made testing more accurate, accessible, and cost-effective, fueling demand across both clinical and research settings. Furthermore, government initiatives and funding for genomic research and healthcare improvements, along with an increased focus on preventative care and early diagnosis, are accelerating market expansion.
The UK genetic disease diagnostics market is projected to grow during the forecast period. The UK government's commitment to healthcare innovation, including funding for genomic research and initiatives such as the 100,000 Genomes Project, is accelerating the development and application of genetic testing. Moreover, the increasing prevalence of genetic diseases and the growing adoption of direct-to-consumer tests are further driving market expansion. Furthermore, insurance companies are focusing on launching novel products that provide insights into patients' genetic profiles, paving the way for more effective treatment plans. For instance, Bupa, an insurance company, launched Medication Check in July 2025. It is a saliva test that offers personalized guidance on the effectiveness of medicine based on an individual’s genome sequence.
The genetic disease diagnostics market in France is expected to show steady growth over the forecast period, driven by advancements in medical research, an increasing focus on personalized healthcare, and rising awareness of genetic disorders. The French healthcare system is placing a greater emphasis on precision medicine, which is driving demand for genetic disease diagnostics services, particularly in oncology, hereditary diseases, and prenatal testing.
Germany genetic disease diagnostics market is projected to expand during the forecast period. The country's strong healthcare infrastructure and commitment to integrating genomic technologies into clinical practice are driving the adoption of genetic testing, particularly for oncology, rare diseases, and prenatal screening. Government funding for genomics research and the expansion of healthcare initiatives aimed at early disease detection further support market growth.
Asia Pacific Genetic Disease Diagnostics Market Trends
The genetic disease diagnostics market in the Asia Pacific is expected to experience the highest growth rate of 21.3% CAGR during the forecast period. The growing prevalence of chronic and genetic diseases, along with the rising demand for personalized medicine, is fueling the need for these services. Governments in several countries are also supporting the market by funding genomics research and integrating genetic disease diagnostics into public health programs. Furthermore, the increasing adoption of direct-to-consumer genetic disease diagnostics and the expansion of healthcare access in emerging economies are contributing to the overall market growth.
China genetic disease diagnostics market is projected to expand throughout the forecast period, driven by several key factors, including increasing healthcare investments, rising prevalence of genetic disorders, and advancements in medical research. The Chinese government is actively promoting the development of precision medicine and genomics research, which is driving the demand for genetic disease diagnostics services.
The genetic disease diagnostics market in Japan is expected to grow during the forecast period, driven by advancements in genomic research, increasing demand for personalized medicine, and a growing focus on preventive healthcare. The Japanese government has made significant investments in genomic healthcare, promoting initiatives to integrate Genetic Disease Diagnostics into public health programs and clinical practice.
Latin America Genetic Disease Diagnostics Market Trends
The genetic disease diagnostics market in Latin America is expected to experience significant growth throughout the forecast period. There is a growing demand for genetic disease diagnostics services, particularly in countries with stronger healthcare infrastructure, such as Brazil and Argentina. The expanding adoption of personalized medicine, coupled with government initiatives to enhance healthcare access and funding for genomic research, is further supporting the market. Moreover, the rising middle class, increasing healthcare investments, and greater access to direct-to-consumer genetic tests are fueling market expansion across the region.
Brazil genetic disease diagnostics market is anticipated to grow during the forecast period, primarily driven by increasing demand for personalized medicine, rising awareness of genetic disorders, and advancements in healthcare technology.
MEA Genetic Disease Diagnostics Market Trends
The genetic disease diagnostics market in the MEA is significantly growing, driven by falling sequencing costs, government genomics programmes, rising NCD/cancer burden, premarital/carrier screening demand, and private-sector investment. Countries such as the UAE and Saudi Arabia are focusing on partnerships, expanding local players, and increasing lab capacity, whereas smaller markets such as Bahrain, Oman, and Kuwait focus on growing public programmes and hospital lab investments
Saudi Arabia genetic disease diagnostics market is anticipated to experience substantial growth during the forecast period. Saudi Arabia's government is actively supporting genomic research and the integration of genomic technologies into healthcare systems to enhance early disease detection and personalized treatment. With a growing incidence of genetic disorders and chronic diseases, there is an increasing demand for genetic testing, particularly for prenatal screening, oncology, and rare diseases.
Key Genetic Disease Diagnostics Company Insights
The global genetic disease diagnostics industry is highly competitive, with numerous players striving to maintain market leadership through innovation, product diversification, and strategic investments. These companies boast extensive product portfolios, a broad geographic presence, and robust distribution networks, allowing them to cater to a wide range of customer needs across both clinical and direct-to-consumer markets. To stay ahead of the competition, many industry leaders have made substantial investments in research and development, enabling them to introduce advanced and cutting-edge genetic disease diagnostics solutions. These efforts not only enhance their market position but also drive the continuous evolution of the industry, meeting the growing demand for personalized medicine, early disease detection, and genomic research.
Key Genetic Disease Diagnostics Companies:
The following are the leading companies in the genetic disease diagnostics market. These companies collectively hold the largest market share and dictate industry trends.
- 24 genetics
- Circle DNA
- Tellmegen
- 23andme
- AncestryDNA
- MyDNA
- Everly Well
- Igenomix
- VitaGen
- Myriad Genetics, Inc.
- Mapmygenome
- Helix OpCo LLC
- MyHeritage Ltd.
- Illumina, Inc.
- Color Genomics, Inc.
- Amgen, Inc.
- Beyond Nutrition Health and Wellness Services DMCC
Recent Developments
-
In August 2025, Natera introduced Fetal Focus, a non-invasive prenatal test (NIPT) intended to detect fetal mutations for several autosomal recessive single-gene disorders (including cystic fibrosis, spinal muscular atrophy, alpha-thalassemia, and beta-hemoglobinopathies) using a maternal blood sample, even when the biological father’s sample is unavailable.
-
In May 2025, Targeted Genomics announced a commercial collaboration with OraSure Technologies. OraSure is the manufacturer of ORAcollect Dx, the only FDA-approved saliva collection device for OTC use. This collaboration would expand consumer access to the Targeted Genomics at-home celiac genetic test.
-
In May 2025, CENTOGENE announced the launch of its new Reproductive Genetics Portfolio, introducing both Preimplantation Genetic Testing for Aneuploidy (PGT-A) and comprehensive carrier screening services. The PGT-A test, enhanced by CENTOGENE’s database of over one million sequences, offers high sensitivity, diagnostic accuracy, and an optional rapid turnaround of up to 24 hours for IVF embryos. Alongside this, the carrier screening service (CentoScreen) provides coverage of≥99% of 332 genes, available for individuals or couples, enabling the early detection of inherited genetic risks.
-
In December 2024, the UAE Ministry of Health and Prevention (December 2024) announced that starting January 2025, genetic testing will become mandatory in the national premarital screening program for all Emiratis planning to marry. Screening will be expanded to 570 genes associated with over 840 genetic disorders, aiming to reduce the incidence of inherited diseases and strengthen the nation’s genome strategy.
-
In March 2024, BGI Genomics released the NOVA Newborn Genetic Screening Test, assessing risk across 246 genes and 112 genetic diseases (covering 254 subtypes). This advances newborn screening using prenatal carrier screening approaches.
-
In September 2024, KK Women’s & Children’s Hospital in Singapore launched PREDICT (PaREnthood genetic DIsease Carrier Test), the first expanded screening program in Asia targeting “at-risk” couples for severe recessive genetic disorders. The voluntary, free program (2024-2027) targets ~40,000 eligible couples, focusing on early reproductive planning.
-
In October 2024, EpiMedTech announced the launch of epiGeneComplete,a comprehensive profile test that provides insight into aging, metabolic issues, inflammation, stress, and more. The test uses an NGS platform along with DNA methylation & SNP markers for higher accuracy
-
In November 2024, ProPhase Labs, Inc. announced that they have launched DNA Complete, Inc., which will be a wholly owned subsidiary offering D2C DNA tests
-
In November 2024, Myriad Genetics, Inc. launched SneakPeek, an at-home gender test, available across 8,800 retailers in the U.S.
Genetic Disease Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 14.26 billion
Revenue forecast in 2033
USD 57.83 billion
Growth rate
CAGR of 19.1% from 2025 to 2033
Actual data
2021 - 2024
Forecast period
2025 - 2033
Quantitative units
Revenue in USD million/billion and CAGR from 2025 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Technology, application, product, channel, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; Germany; UK; France; Spain; Italy; Denmark; Sweden; Norway; China; Japan; India; South Korea; Australia; Thailand; Brazil; Argentina; Saudi Arabia; Kuwait; UAE; South Africa
Key companies profiled
24 genetics; Circle DNA; Tellmegene; 23andme; AncestryDNA; MyDNA; Everly Well; Igenomix; VitaGen; Myriad Genetics Inc.; Mapmygenome; Helix OpCo LLC; MyHeritage Ltd.; Illumina, Inc.; Color Genomics, Inc.; Amgen, Inc.; Beyond Nutrition Health and Wellness Services DMCC
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Genetic Disease Diagnostics Market Report Segmentation
This report forecasts revenue growth at global, regional & country levels and provides an analysis of the industry trends in each of the sub-segments from 2021 to 2033. For this study, Grand View Research has segmented the global genetic disease diagnostics market report based on technology, application, product, channel, end use, and region:
-
Technology Outlook (Revenue, USD Million, 2021 - 2033)
-
Next Generation Sequencing
-
Array Technology
-
PCR-based Testing
-
FISH
-
Others
-
-
Application Outlook (Revenue, USD Million, 2021 - 2033)
-
Ancestry & Ethnicity
-
Traits Screening
-
Genetic Disease Carrier Status
-
New Baby Screening
-
Health and Wellness-Predisposition/Risk/Tendency
-
-
Product Outlook (Revenue, USD Million, 2021 - 2033)
-
Consumables
-
Equipment
-
Software & Services
-
-
Channel Outlook (Revenue, USD Million, 2021 - 2033)
-
Online
-
Offline
-
-
End Use Outlook (Revenue, USD Million, 2021 - 2033)
-
Hospitals & Clinics
-
Diagnostic Laboratories
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2021 - 2033)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global genetic disease diagnostic market size was estimated at USD 11.71 billion in 2024 and is expected to reach USD 14.25 billion in 2025.
b. The global genetic disease diagnostic market is expected to grow at a compound annual growth rate of 19.1% from 2025 to 2033 to reach USD 57.83 billion by 2033.
b. North America genetic disease diagnostics market dominated and accounted for a 44.49%% share in 2024. The rising demand for diagnostic services is largely driven by the growing prevalence of chronic illnesses and the advancement of diagnostic technologies.
b. Some key players operating in the genetic disease diagnostic market include 24 genetics; Circle DNA; tellmegene; 23andme; AncestryDNA; MyDNA; Everly Well; Igenomix; VitaGen; Myriad Genetics Inc.; Mapmygenome; Helix OpCo LLC; MyHeritage Ltd.; Illumina, Inc.; Color Genomics, Inc.; Amgen, Inc.; Beyond Nutrition Health and Wellness Services DMCC
b. Key factors that are driving the genetic disease diagnostic are driven by technological advancements, increasing demand for personalized medicine, and growing demand for newborn screening.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










