- Home
- »
- Clinical Diagnostics
- »
-
In-vitro Diagnostics Enzymes Market Size Report, 2030GVR Report cover
![In-vitro Diagnostics Enzymes Market Size, Share & Trends Report]()
In-vitro Diagnostics Enzymes Market Size, Share & Trends Analysis Report By Enzyme Type (Polymerase & Transcriptase, Proteases), By Disease Type, By Technology Type, By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68039-246-6
- Number of Report Pages: 199
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
In-vitro Diagnostics Enzymes Market Trends
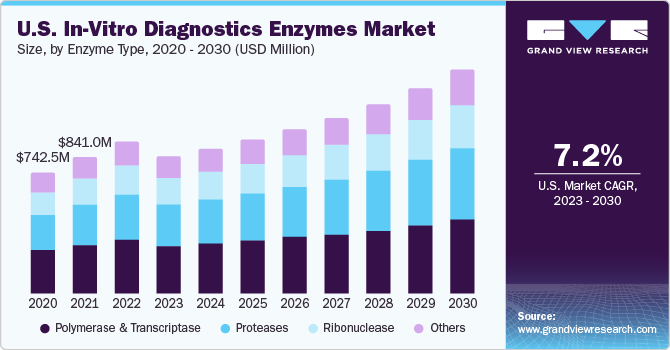
The global in-vitro diagnostics enzymes market size was valued at USD 2.39 billion in 2022 and is anticipated to grow at a compound annual growth rate (CAGR) of 7.9% from 2023 to 2030. Enzymes are adopted in the diagnosis of various metabolic disorders owing to their remarkable catalytic properties. Several research studies have represented the clinical application of enzymes, such as alanine transaminase, acid phosphatase, creatine kinase, aspartate transaminase, lactate dehydrogenase, and gelatinase-B. These enzymes are preferred biomarkers in several disease conditions, including myocardial infarction, liver disease, schizophrenia, renal disease, rheumatoid arthritis, and cancer.
Various molecular biology techniques are continuously gaining popularity as in-vitro diagnostics (IVD) for the detection and prevention of several diseases. Among these techniques, the polymerase chain reaction (PCR) had a greater impact on the practices involved in the molecular biology field. PCR implements a thermostable polymerase to produce multiple copies of a specific nucleic acid region rapidly and exponentially. Taq polymerase, reverse transcriptase enzyme, and thermostable DNA polymerase are the commonly used enzymes during PCR amplification cycles.
The utility of enzymes for the diagnosis of COVID-19 has spurred the market growth. A wide range of commercial COVID-19 tests were available in the marketplace. Some of the tests either detect the SARS-CoV-2 viral RNA using the nucleic acid hybridization-related strategies or PCR technique while others are serological and immunological assays that detect the antibodies produced in response to the virus.
The PCR method is highly accepted to test the presence of the SARS-CoV-2 virus. This method uses a DNA-copying enzyme, Taq DNA polymerase, which remains active at high temperatures. Along with this, reverse transcriptase is used to copy the RNA of the virus in the DNA for further amplification. Creative Enzymes and BioVendor are key companies that offer enzymes for the diagnosis of COVID-19.
Enzyme Type Insights
Polymerase and transcriptase accounted for the largest revenue share of 36% in 2022. This is attributed to their wide applications in molecular diagnostic assays. In addition, the presence of a substantial number of players that offer these enzymes is expected to enhance the segment growth. In June 2023, AIST and Asahi Kasei Pharma collaborated on the Smart Cell Project of NEDO to improve cholesterol esterase production efficiency, an enzyme utilized in vitro diagnostic assays enabling the manufacture of a commercial product called CEN II.
The proteases segment is expected to grow at the fastest CAGR of 8.3% during the forecast period. Proteases are expected to gain traction in common molecular biology procedures. The application of heat-labile protease is used to digest the heat-stable molecular biology enzymes, such as PvuII and Taq Polymerase, which further inactivates due to a mild heat treatment.
This indicates the major benefit of protease in instances where further chemical reactions can be completed without the requirement of an intermediate purification stage; therefore, it reduces product loss and saves time. This benefit of protease makes it an important diagnostic enzyme to be used in IVD procedures.
The use of proteases in the oncology disease type is expected to witness significant growth throughout the forecast period with the rising cases of cancer globally and the expansion of IVD techniques, such as In Situ Hybridization (ISH) and Next-Generation Sequencing (NGS), in the field of oncology.
Disease Type Insights
Infectious disease accounted for the largest revenue share amongst other disease types. The wide applicability of PCR technology in infectious disease diagnosis has led to early diagnosis and treatment. Organisms that are difficult to be detected can now be identified with better precision and sensitivity. The application of PCR in infectious disease diagnosis includes the detection of tuberculosis, streptococcal pharyngitis, atypical pneumonia, ulcerative urogenital infections, and several persistent infections.
The Thermus thermophilus DNA polymerase gene expressed in Escherichia coli allows an efficient reverse transcriptase activity for the detection of cellular mRNA expression in a single step. Moreover, an IgM antibody capture ELISA (MAC-ELISA) is a proven technology for dengue diagnosis during the early convalescent or late acute phase of the infection. Platelia Dengue NS1 antigen-capture ELISA kits and Panbio Dengue Duo IgG and IgM Rapid Cassette test kits are some of the enzyme-based kits for the diagnosis of infectious diseases.
The oncology segment is expected to expand at the fastest CAGR over the forecast period. Large-scale adoption of ISH methods as well as the development of high-throughput technologies, such as next-generation DNA sequencing and comparative genomic hybridization, for the diagnosis of human tumors, drive the usage of diagnostic enzymes in the oncology segment.
Technology Type Insights
Histology assays dominated the in-vitro diagnostics enzymes market with a significant revenue share owing to the advantages offered by the technique in the analysis of tissue samples for diagnostic purposes. Moreover, histopathology is quick, with little or no risk to patients, and helps clinicians analyze the internal architecture of tissues and cells.
Molecular diagnostics is preferred over serology for the diagnosis of infectious diseases as this technique is highly sensitive and offers the detection of disease-causing agents, allowing early detection of several disorders. The molecular diagnostics segment is expected to witness the fastest growth over the forecast period. Moreover, enzymes are crucial to multiple steps in molecular diagnostic assays, such as NGS library and sample preparation. They provide shortcuts for difficult or slow reactions and enable repair, modification, and amplification of nucleic acids for a wide range of applications.
Enzymes form an essential component of clinical chemistry diagnostic assays owing to the specificity and speed offered by enzymes. Enzyme specificity is used either for the removal of interferents in another reaction or for the measurement of a substrate in an assay. Enzymes are also used to measure activators, inhibitors, or cofactors. In addition, the catalytic activity of enzymes makes them ideal for use as labels in immunoassay techniques. Thus, key players, such as BBI Solutions, offer a wide range of enzymes for clinical chemistry use. In November 2022, New England Biolabs introduced Induro Reverse Transcriptase, a novel cDNA synthesis enzyme that overcomes RNA-sequencing barriers and is ideal for challenging synthesis from long transcripts, strong secondary structures, and inhibitor-inhibited samples.
End-use Insights
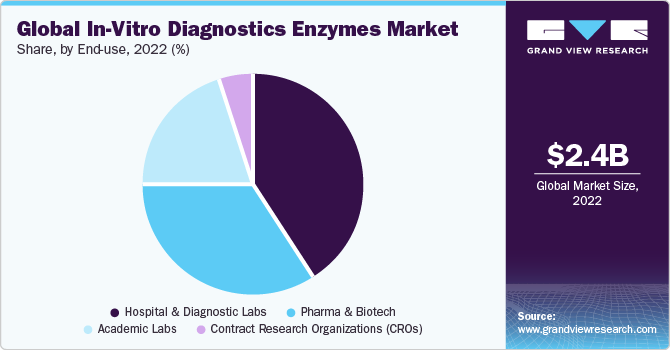
The hospitals and diagnostic laboratories segment dominated the market with the largest revenue share of 41.6% in 2022. The usage of IVD assays in hospitals has increased over the years. In several hospitals and clinics, physicians are switching to histopathology and molecular diagnostics from conventional testing procedures. The long turnover time associated with the conventional processes is boosting the adoption rate of histopathology-based tests as these aid in reducing the timelines.
The academic labs segment is expected to expand at the fastest CAGR of 9.1% during the forecast period. An increase in the application of NGS and PCR technologies in academic and institutional projects drives the adoption rate of diagnostic enzymes used during the IVD procedures conducted in these settings.
For instance, researchers at the UNC Medical Center Microbiology and Molecular Microbiology Laboratories, in the U.S., explored the usage of NGS technology for accurate COVID-19 diagnosis in March 2020. The use of this technology helped scientists in the identification of the mutations and gain insights related to the genomic code of COVID-19 causing virus. This application of NGS drives the utilization of enzymes used in library preparation, such as T4 polynucleotide kinas, ligase, DNA polymerase, and reverse transcriptase.
Regional Insights
North America dominated the largest revenue share of 41.7% in 2022 owing to the high demand for IVD enzymes and the increasing prevalence of infectious disorders in the region. To combat the ongoing COVID-19 pandemic, the FDA has issued emergency use authorizations for in-vitro COVID-19 laboratory-developed tests.
In addition, most of these emergency use authorizations are Enzyme-Linked Immunosorbent Assay (ELISA) and Reverse Transcription Polymerase Chain Reaction (RT-PCR)-based tests for the detection of SARS-CoV-2. Owing to the high usability of enzymes during RT-PCR, the development of such tests is expected to generate revenue in the North American market.
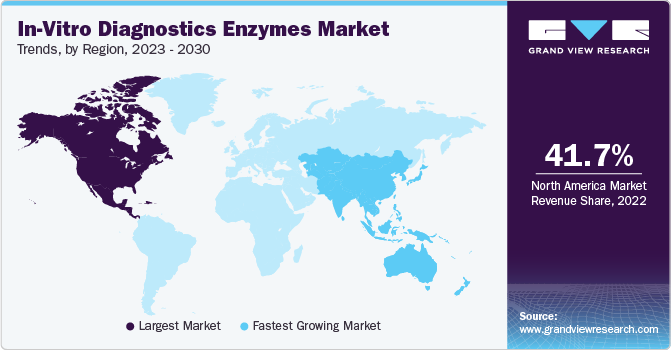
Asia Pacific is expected to grow at the fastest CAGR of 9.2% during the forecast period due to the improvements in healthcare infrastructure, the introduction of insurance policies allowing favorable reimbursements, and the increasing prevalence of cancer and HIV/AIDS, leading to increased demand for diagnostic tests. China holds a significant position in the Asia Pacific market due to the presence of several suppliers of enzymes.
Key Companies & Market Share Insights
Key companies are making continuous efforts to fulfill the demand for enzymes utilized during diagnostic procedures for clinical disorders, especially COVID-19. For instance, in May 2020, Richcore Lifesciences, an Indian company, along with the Indian Institute of Science Education and Research at Pune and Chandigarh and the Indian Institute of Science, Bengaluru, produced and optimized Reverse Transcriptase and Taq Polymerase, two key enzymes, for the RT-PCR diagnostic kits. The company is currently offering these enzyme samples to test kit manufacturers to approve the consistency and stability of enzymes. Once approved, the company would be able to mass-produce these enzymes for millions of certified testing kits in India. Such initiatives undertaken by the companies drive large-scale production of enzymes to address the urgent need for testing kits for COVID-19 diagnosis. In January 2023, Thermo Fisher Scientific acquired The Binding Site Group, a global specialty diagnostics leader, in an all-cash transaction valued at £2.3 billion. The acquisition expands Thermo Fisher's specialty diagnostics portfolio with pioneering innovation in multiple myeloma diagnostics and monitoring. It aims to enable further advancements in patient outcomes.
Key In-vitro Diagnostics Enzymes Companies:
- Merck KGaA
- Codexis, Inc.
- F. Hoffmann-La Roche Ltd.
- Amano Enzyme Inc.
- Advanced Enzymes Technologies Ltd.
- Biocatalysts Ltd.
- Amicogen
- Dyadic International
- BBI Solutions
- Affymetrix
- American Laboratories
In-vitro Diagnostics Enzymes Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 2.18 billion
Revenue forecast in 2030
USD 3.70 billion
Growth Rate
CAGR of 7.9% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
December 2023
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Enzyme type, disease type, technology type, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Key companies profiled
Merck KGaA; Codexis, Inc.; F. Hoffmann-La Roche Ltd.; Amano Enzyme Inc.; Advanced Enzymes Technologies Ltd.; Biocatalysts Ltd.; Amicogen; Dyadic International; BBI Solutions; Affymetrix; American Laboratories
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global In-vitro Diagnostics Enzymes Market Report SegmentationThis report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global in-vitro diagnostics enzymes market report based on enzyme type, disease type, technology type, end-use, and region:
-
Enzyme Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Proteases
-
Polymerase & Transcriptase
-
Ribonuclease
-
Others
-
-
Disease Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Infectious Disease
-
COVID-19 Testing
-
Hepatitis
-
HIV
-
Others
-
-
Diabetes
-
Oncology
-
Cardiology
-
Nephrology
-
Autoimmune diseases
-
Others
-
-
Technology Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Histology Assays
-
Molecular Diagnostics
-
PCR Assays
-
NGS Assays
-
Others
-
-
Clinical Chemistry
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Pharma & Biotech
-
Hospital & Diagnostic Labs
-
Contract Research Organizations (CROs)
-
Academic Labs
-
-
Regional Outlook (Revenue in USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Norway
-
Denmark
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global in-vitro diagnostics enzymes market size was estimated at USD 2.39 billion in 2022 and is expected to reach USD 2.18 billion in 2023.
b. The global in-vitro diagnostics enzymes market is expected to grow at a compound annual growth rate of 7.9% from 2023 to 2030 to reach USD 3.70 billion by 2030.
b. North America dominated the IVD enzymes market with a share of 41.70% in 2022. This is attributable to the high demand for IVD enzymes and the increasing prevalence of infectious disorders in the region.
b. Some of the key players in the IVD enzymes market include Merck KGaA, Codexis, Inc., F. Hoffmann-La Roche Ltd., Amano Enzyme Inc., Advanced Enzymes Technologies Ltd., Biocatalysts Ltd., Amicogen, Dyadic International, BBI Solutions, Affymetrix, American Laboratories
b. Key factors driving the IVD enzymes market growth include a rise in the adoption of IVD in clinical diagnostics.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





