The GCC's Genomics Inflection Point: Embedding Advanced Therapeutics into National Health and Industrial Policy
The Middle East is entering a pivotal policy and investment cycle in healthcare transformation. After sustaining public spending on national genome programs, digital health infrastructure, and precision medicine initiatives, regional leaders are now shifting focus from pilot projects to system-wide integration and scalable value creation. Genomics and gene therapies are no longer peripheral innovation themes; they are being embedded into national health strategies, industrial policies, and long-term economic diversification agendas.
Governments in the GCC increasingly view genomics as a strategic asset to enhance population health outcomes, reduce the economic burden of chronic disease, and catalyze high-value biopharmaceutical manufacturing. Investments are expanding from data generation to building end-to-end capabilities in clinical genomics, cell and gene therapy development, and GMP-compliant biomanufacturing. This creates a strong pipeline of opportunities for global biotechs, CDMOs, technology providers, and institutional investors seeking to participate in a rapidly maturing market.
At the same time, policymakers recognize that realizing the full return on these investments requires addressing underlying structural issues: harmonized regulation, sustainable reimbursement models, interoperable data ecosystems, and advanced workforce development. The strategic priority is now to align health, industrial, and innovation policies to support scale, foster public–private partnerships, and de-risk investment in local manufacturing and R&D.
With targeted reforms and coordinated investment, the Middle East is well positioned to emerge as a regional hub for genomics-driven healthcare and advanced therapies—offering investors a compelling combination of political commitment, funding capacity, and unmet clinical need.
Emerging Opportunities in Genomic Medicine and Advanced Therapies in the GCC
-
Growing National Genome Initiatives
The UAE, Saudi Arabia, and Qatar have each launched ambitious national genome programs, creating rich repositories of diverse genomic data. The Emirati Genome Programme has already sequenced over 800,000 genomes, laying a strong foundation for future cross-border collaboration and data integration. Saudi Arabia's Human Genome Program has catalogued more than 7,500 genetic variants, opening new avenues for the development of region-specific precision therapies. As regional interoperability and standardized data-sharing frameworks evolve, the GCC stands poised to lead in population-wide genomic reference databases, accelerating innovation in precision therapy and clinical trial landscapes. -
Potential for Expansion in Local Manufacturing
With the growing demand for cell and gene therapy (CGT), the Middle East offers significant opportunities to develop domestic manufacturing capabilities for viral vectors, plasmids, and CAR-T components. Building GCC-based GMP infrastructure can reduce reliance on overseas supply chains, enable faster therapy delivery, and create a competitive environment for cost-effective treatment options. Enhanced local production will strengthen supply stability, lower logistics costs, and improve treatment accessibility for hospitals and patients. -
Advancing Clinical Delivery and Workforce Development
As gene therapy adoption expands, the region has a chance to invest in specialized training across multidisciplinary clinical teams. Increasing numbers of genetic counselors and equipping more centers for advanced therapy medicinal products (ATMPs) will empower the GCC to match international standards for patient access and care quality. Targeted talent development and knowledge sharing will support program expansion, enhance clinical readiness, and ensure robust long-term patient monitoring—positioning the GCC as a leader in advanced clinical services. -
Evolving Regulatory and Reimbursement Systems
Advanced therapies present an opportunity to modernize regulatory and funding frameworks across the GCC. Initiatives to establish long-term safety registries and harmonize approval processes will strengthen the region’s capacity for innovation and cross-border clinical trials. Adoption of value-based payment mechanisms and data-driven outcome tracking can foster payer confidence and boost market uptake for transformative therapies such as Zolgensma and Elevidys. As regulatory alignment progresses, the GCC is set to create a supportive environment for breakthrough therapies benefiting patients and healthcare systems alike.
From Vision to Implementation: Advancing Cell and Gene Therapy Capabilities Across the Middle East
Despite these challenges, the past 18 months have produced meaningful progress. The region is moving from fragmented initiatives to a more connected precision-medicine ecosystem.
-
Manufacturing and Technology Transfer
The 2025 EVA Pharma partnership with Porton Advanced marks a significant step toward regional localization of viral vector and CAR-T manufacturing. Lentiviral production capacity in Egypt and Saudi Arabia reduces reliance on imports, thereby strengthening supply security.
-
National Biotechnology Strategies
Saudi Arabia’s 2024 multi-ministry MoU with Vertex Pharmaceuticals signals a policy shift toward domestic gene therapy R&D and manufacturing, reflecting Vision 2030’s drive for sovereign capabilities in advanced therapeutics.
-
Integrated R&D and Clinical Trial Platforms
The Abu Dhabi DoH–M42–PureHealth partnership with GEMMABio (2025) illustrates a fully integrated ecosystem. By combining R&D, manufacturing, and clinical trials, the platform supports early SMA1 trials and sets the stage for future rare disease programs.
-
Clinical Delivery at Scale
Qatar’s Sidra Medicine has treated 45 children with SMA and DMD gene therapies, operationalizing early cases of Elevidys and Zolgensma. Alignment of diagnostics, clinical readiness, and international standards enables the delivery of advanced treatment at scale.
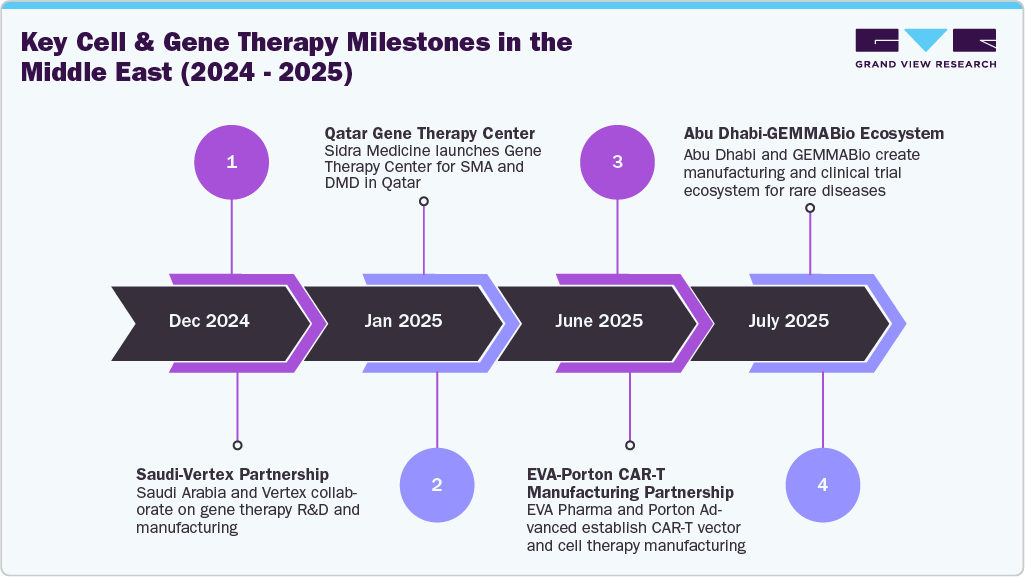
These developments demonstrate that regional momentum exists across manufacturing, regulation, R&D, and clinical delivery, providing a strong foundation for actionable strategic recommendations.
Strategic Pathways and Recommendations for Sustainable Regional Growth
Considering the current challenges and recent developments, the Middle East should prioritize five strategic pathways to build a sustainable and scalable genomics–gene therapy ecosystem.
- Build Regional Biomanufacturing Capacity and Shared CDMO Models
Sustainable access to advanced therapies requires reliable and affordable production of vectors, plasmids, and cell therapy inputs. Establishing multi-country biomanufacturing networks with shared GMP standards, centralized quality oversight, and coordinated demand forecasting will reduce duplication and costs. Regional contract development and manufacturing organizations can support viral vector, CAR-T, and future gene-editing platforms, enabling supply chain resilience and scalability.
- Embed Genomics Across the Patient and R&D Journey
Genomics should evolve from isolated national programs into actionable clinical tools. This includes linking population sequencing with newborn screening, rare disease registries, clinical trial recruitment pathways, and algorithms for determining therapy eligibility. Health systems must prioritize interoperability, cross-border governance, and integration with electronic health records to reduce diagnostic delays and improve trial readiness.
- Establish Harmonized Regulatory and Value-Based Access Frameworks
Advanced therapies challenge traditional regulatory and reimbursement models. GCC-wide alignment on gene therapy approval processes, long-term safety registries, and outcomes-based payment structures will accelerate access, strengthen payer confidence, and reduce inefficiencies. Embedding real-world evidence platforms into regulatory and reimbursement cycles ensures ongoing validation of therapy durability and cost-effectiveness.
- Develop Multidisciplinary Talent Pipelines and Clinical Readiness Programs
Human capital is the most significant constraint on regional CGT scale-up. Structured pipelines are essential for genetic counselors, bioinformaticians, GMP technologists, clinical pharmacists, CAR-T preparation teams, and long-term monitoring units. Shared academic hubs in Abu Dhabi, Doha, Riyadh, and Cairo can serve as centers for training, competency certification, and operational readiness, providing a platform for collaboration and the exchange of expertise.
- Create Integrated Precision-Medicine Ecosystems
The region should transition from independent initiatives to fully integrated clusters that link genomics, manufacturing, R&D, clinical trials, and commercialization. Precision-medicine zones and biotech parks can pool infrastructure, accelerate technology transfer, foster partnerships, and optimize patient pathways. Integrated ecosystems position the Middle East as a creator of advanced therapeutics rather than a passive consumer.
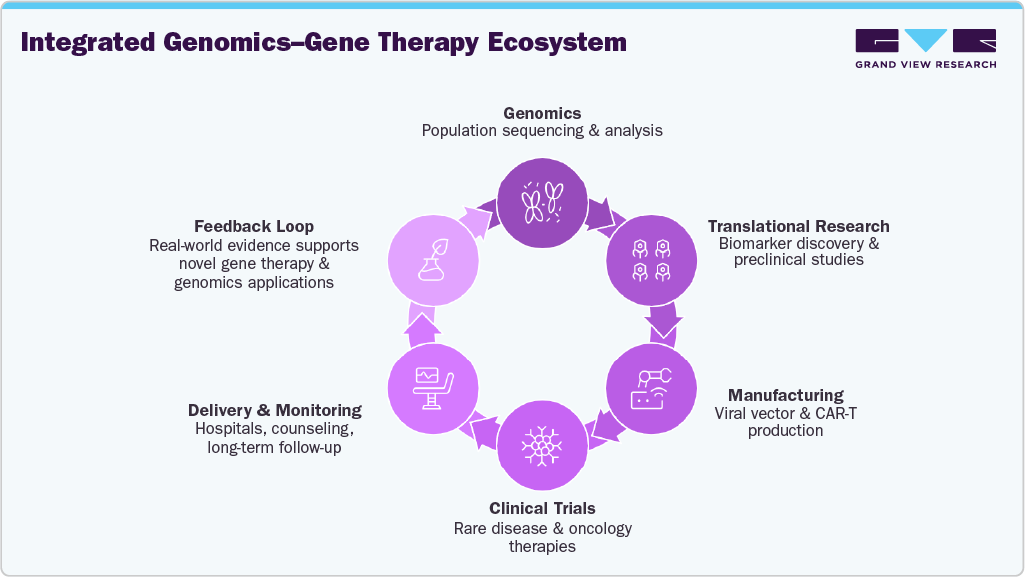
Key Takeaways:
-
Integration is essential: Genomics and gene-based therapies must be developed together rather than in isolation to achieve meaningful clinical and economic impact.
-
Localization drives sustainability: Regional manufacturing of vectors, plasmids, and CAR-T products is critical for cost control, supply resilience, and long-term therapy access.
-
Harmonized frameworks accelerate adoption: Regulatory alignment, outcomes-based reimbursement, and real-world evidence platforms are foundational for scalable patient access.
-
Human capital is the backbone: Skilled clinicians, bioinformaticians, GMP technologists, and genetic counselors are the most critical constraint to ecosystem growth.
-
From consumer to creator: By building integrated clusters that link genomics, manufacturing, R&D, clinical trials, and commercialization, the Middle East is positioning itself as a regional and global contributor to advanced therapeutic innovation.