- Home
- »
- Medical Devices
- »
-
Clinical Trial Supplies Market Size And Share Report, 2030GVR Report cover
![Clinical Trial Supplies Market Size, Share & Trends Report]()
Clinical Trial Supplies Market Size, Share & Trends Analysis Report By Clinical Phase (Phase I, Phase II, Phase III), By Product & Services, By End-Use, By Therapeutic Use, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-1-68038-514-4
- Number of Report Pages: 123
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2023
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Market Size & Trends
The global clinical trial supplies market size was estimated at USD 2.58 billion in 2023 and is anticipated to grow at a compound annual growth rate (CAGR) of 6.5% from 2024 to 2030. Globalization, and rise in the number of biologics & biosimilar drugs in clinical trials are among the major factors expected to drive the market growth. Rapid adoption of a supply chain management system to surmount R&D expenditure pressure and increase operational efficiency, as clinical trial supplies account for a large share of the total R&D expenditure of biopharmaceutical companies, is anticipated to propel market growth in near future. There has been a significant rise in biologics and temperature-sensitive drugs in clinical trials.
Most clinical trials are currently being conducted in developing economies. The increasing cost of clinical trials and complications in the recruitment of patients have encouraged biopharmaceutical companies to outsource clinical trials to regions such as Asia Pacific, Latin America, Central & Eastern Europe, and the Middle East. Disease variation in developing economies further aids biopharmaceutical companies in performing clinical trials on rare diseases. Some regions, such as Asia Pacific, also provide greater economic benefits to biopharmaceutical companies, as governments in Singapore and China allocate funds to promote biomedical research. In Latin America, patient recruitment is easy due to reduced language barriers, which can help obtain informed consent easily, resulting in a faster clinical trial process.
There has been a significant investment in supply chain management software by clinical trial supply providers. Growing complexity in clinical trials and increased competition among market players are factors responsible for adopting new technologies in supply chain management. The need for software for inventory management, supply chain planning, and ancillary supply chain management is expected to grow, owing to industry players' increasing adoption of new technologies. For instance, in pharmaceutical manufacturing, the application of digital twin technology facilitates the expedited development of drugs, which is achieved by using real-world data to generate simulations within a laboratory setting, enabling scientists to anticipate responses to biological processes under specific circumstances.
Market Concentration & Characteristics
The market growth stage is medium, and the pace of market growth is accelerating. The clinical trial supplies market is characterized by a high degree of innovation. Continuous advancements in novel clinical trial equipment, such as state-of-the-art imaging systems, monitoring devices, and diagnostic tools, enhance overall efficiency in clinical trials, accelerating market demand.
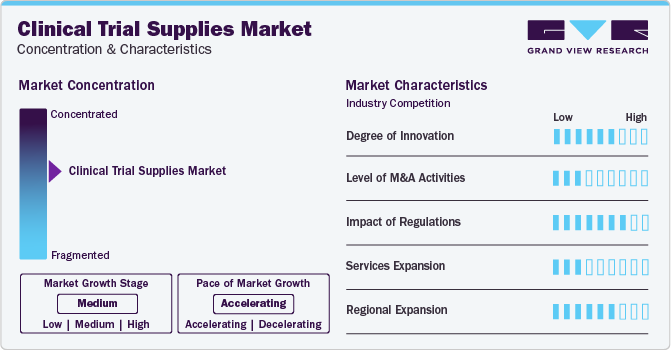
The clinical trial supplies market is also characterized by a leading player's medium level of merger and acquisition (M&A) activity. This market space witnesses many acquisitions to provide a full spectrum solution to the sponsors. As clinical trials become increasingly global, companies may seek to expand their geographic reach to conduct such trials in various regions.
The clinical trial supplies market is heavily regulated, which are enforced by agencies such as the U.S. Food & Drug Administration (FDA) and European Medicines Agency (EMA) to ensure the safety and rights of participants. In addition, companies in this industry often focus on regional expansion to establish a presence in multiple geographic areas and meet the varying regulatory requirements that come with operating in different regions.
Clinical Phase Insights
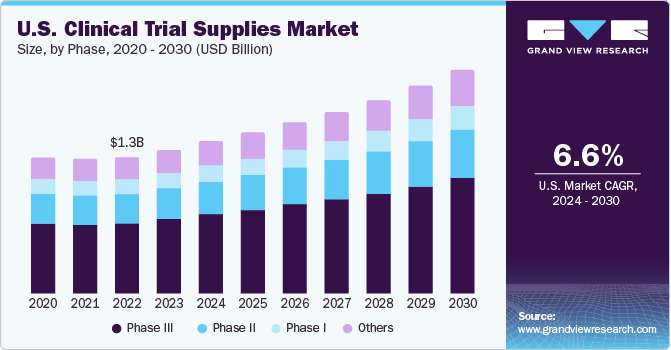
Phase III led the market and accounted for 52.75% of the global revenue in 2023. Phase III clinical trials are more complex when compared to other phases. The list of FDA-approved phase III drugs is comparatively smaller, and the complexity associated with this phase is the highest. The failure rate in this phase is also the highest as the sample size and study design require complex dosing at an optimum level. Loss associated with failure is with respect to human and financial issues, and most failures are due to non-compliance with safety & efficacy standards. Such a scenario is expected to surge the demand for efficient clinical trials supply and logistics, which, in turn, is expected to impact market growth positively.
Phase I clinical trials are anticipated to register the fastest CAGR of 7.0% during the forecast period. A small sample population and high capital investment are major factors responsible for outsourcing most clinical trials. Increase in the number of phase I clinical trials being outsourced and the globalization of clinical trials are expected to drive the market for phase I clinical trial supplies.
Product & Service Insights
Supply chain management accounted for the largest market revenue share in 2023.This scenario exists in most regions worldwide, except in the U.S., wherein the manufacturing segment is also expected to grow at a lucrative rate. The recent COVID-19 pandemic led to a wide disruption in the supply chain along with its impact on the lives of the citizens. This propelled the U.S. to become even more self-reliant, eventually focusing more on manufacturing services. The product/service segment for the clinical trial supplies industry includes several processes, from drug development to logistics to distribution. Based on the type of products & services, the clinical trial supplies industry is divided into three major categories, which comprise all the aspects of clinical trial supplies. These include manufacturing, storage and distribution, and supply chain management.
The manufacturing segment is anticipated to witness significant growth at a CAGR of over 6.6% during the forecast period. Rise in number of clinical trials has resulted in high demand for material supplies, which further increases the demand for quality drugs. Complex molecules and high demand for biologics are expected to boost the manufacturing segment of this industry. Many material supplies are outsourced, which drives the demand for efficient clinical trial supplies. Outsourcing in manufacturing can be attributed to the introduction of new technologies to manufacture complex molecules and an increase in demand for developing cost-efficient products.
Therapeutic Use Insights
Oncology led the market in 2023 and is attributable to presence of a huge R&D pipeline. Majority of oncology drugs require temperature-sensitive distribution, which is expected to fuel the demand for cold chain distribution. Oncology clinical trials are designed to diagnose, manage, and treat cancer & associated symptoms. Clinical trial supplies in oncology include primary and secondary packaging. The primary objective of packaging is to improve patient compliance. Packaging must protect vials from leakage and gases from aerosolizing.
On the other hand, cardiovascular disease trials is anticipated to register a 6.9% growth over the forecast period. Cardiovascular diseases are known to be the leading cause of death globally. According to the CDC, cardiovascular disease claims a life in the U.S. approximately every 33 seconds. In the year 2021, around 695,000 individuals died due to heart disease in the country, constituting 20% of all recorded deaths.
End-use Insights
Pharmaceuticals accounted for the largest market share of 42.48% in 2023. Pharmaceutical companies spend substantial investments in R&D to create novel medications and treatments. To acquire information about the efficacy and safety of their products, clinical trials are an essential step in a drug development process. Therefore, based on end-use, clinical trial supplies industry is segmented into pharmaceuticals, biologics, medical devices, and others.
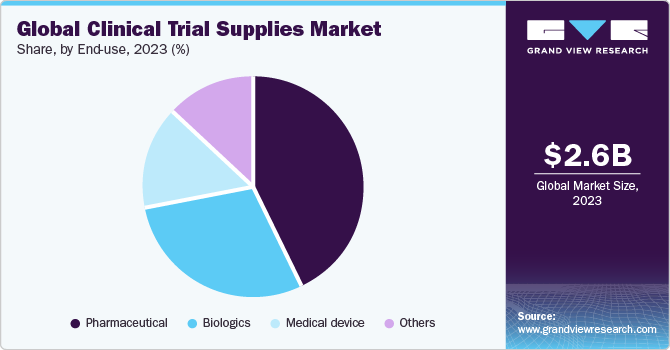
There has been a steady decrease in pharmaceutical drugs in the R&D pipeline as it has been substituted by biological drugs, which is expected to have a slightly negative impact on segment growth during the forecast period. Pharmaceutical drugs are slowly being replaced by biologics, especially in category of topmost innovative drugs. Although pharmaceutical drugs account for the highest number of drugs in clinical trial segment, growing number of biological drugs is likely to have an impact on this number. However, demand for safe, efficacious, and cost-effective medicines is expected to fuel the development of enhanced pharmaceutical drugs, thereby propelling segment’s growth.
Biologics is anticipated to register the fastest growth of 6.8% during the forecast period owing to increased demand for safe and efficacious drugs with lesser adverse effects. An increase in the incidence of cancer and chronic diseases is also expected to fuel the growth of biologics in clinical trials. In addition, increase in biologics in the clinical trial segment is further expected to drive the cold storage and distribution market. It is also expected to increase adoption of newer technologies for better supply chain management.
Regional Insights
North America accounted for the largest market share of 55.49% in 2023. The region conducts the maximum number of clinical trials amongst all, which is a major driver for clinical trial supplies industry growth. Moreover, presence of key players, coupled with advanced technology penetration are major factors responsible for the dominance of this region.
The U.S. clinical trial supplies market is anticipated to witness significant growth rate over the forecast period. Major CROs such as Quintiles; Covance, Inc.; and PAREXEL International Corporation are situated in this region, which is also a driving factor for clinical trial supplies market growth. Demand for reducing R&D cost is changing the preference to emerging countries, thereby increasing demand for cost-effective supplies, which is anticipated to contribute to the growth of clinical trial supplies market in the U.S. The trend of shifting clinical trials sites outside the U.S is expected to continue due to the rising cost of R&D & patient recruitment.
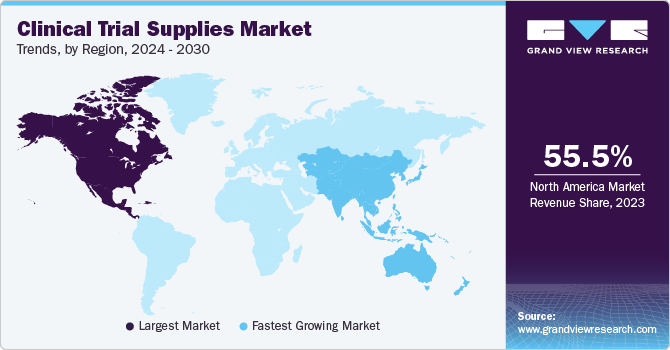
Asia Pacific is expected to be one of the fastest-growing regions with tremendous growth in clinical research and is expected to drive growth of clinical trial supplies market in a region, thereby contributing to a growth of global clinical trial supplies market. Primary factors driving the growth of clinical research in these regions include low cost per patient in Asia Pacific countries and presence of a diverse group of patients that are easy to recruit.
China is one of the lucrative markets for clinical trial supplies players owing to its diverse pool of patients and growing pharmaceutical market. Logistics and supply chain is a major challenge in the country, which is discouraging major biopharmaceutical companies from conducting trials in China. The country accounts for over 27% of global clinical trials conducted as of 2023 and has exhibited lucrative growth in the past 5 years. Trend is driving entry of major players such as Catalent Pharma Solutions in China, which is expected to contribute to the growth of clinical supplies market in the country.
Key Companies & Market Share Insights
The major players operating across the clinical trial supplies market are focused on the adoption of in-organic strategic initiatives such as mergers, partnerships, acquisitions, etc. For instance, in March 2023, Calyx, an eClinical regulatory services, and solutions provider announced the availability through a simulation of Calyx supply, a clinical trial supply forecasting service available through the company's in-house expert statistical design and trial supplies consultants. Moreover, in April 2021, Catalent expanded capabilities at its clinical supply services facility in Philadelphia to support sponsors developing cell and gene therapies.
Key Clinical Trial Supplies Companies:
- Almac Group
- Biocair
- Catalent Inc.
- KLIFO
- Movianto
- PCI Pharma Services
- Sharp Services, LLC
- Thermo Fischer Scientific Inc.
- Marken
- PAREXEL International Corporation
Recent Developments
-
In February 2023, Catalent completed a USD 2.2 million expansion of its clinical supply facility in Singapore. This expansion has enlarged the site's footprint to 31,000 square feet, providing room for installing 35 new freezers dedicated to ultra-low temperature (ULT) storage.
-
In January 2023, ASLAN Pharmaceuticals and Thermo Fisher Scientific entered into a partnership to manufacture a high concentration formulation of Eblasakimab for upcoming studies. Thermo Fisher Scientific will contribute its expertise in biologic manufacturing and scale-up capacity to oversee a clinical supply of Eblasakimab for the anticipated Phase 3 studies.
-
In July 2023, Almac Sciences announced the opening of a custom-built GMP warehouse and dispatch hub at Almac Group’s global headquarters in Craigavon, UK. The facility will support all the manufacturing and lab activities of Active Pharmaceutical Ingredients from development to their commercialization.
Clinical Trial Supplies Market Report Scope
Report Attribute
Details
Market size value in 2024
USD2.73 billion
Revenue forecast in 2030
USD 3.97 billion
Growth Rate
CAGR of 6.5% from 2024 to 2030
Actual data
2018 - 2023
Forecast period
2024 - 2030
Quantitative units
Revenue in USD billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Clinical phase, product & service, end-use, therapeutic use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Norway; Denmark; Sweden; India; China; Japan; Australia; Singapore; South Korea; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Almac Group; Biocair; Catalent Inc.; KLIFO; Movianto; PCI Pharma Services; Sharp Services, LLC; Thermo Fischer Scientific Inc.; Marken; PAREXEL International Corporation
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Clinical Trial Supplies Market Report Segmentation
This report forecasts revenue growth at global, regional & country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global clinical trial supplies market report based on clinical phase, product & services, therapeutic use, end-use, and region:
-
Clinical Phase Outlook (Revenue, USD Billion, 2018 - 2030)
-
Phase I
-
Phase II
-
Phase III
-
Other
-
-
Product & Services Outlook (Revenue, USD Billion, 2018 - 2030)
-
Manufacturing
-
Storage & Distribution
-
Cold chain distribution
-
Non-cold chain
-
-
Supply chain management
-
-
End-use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Pharmaceutical
-
Biologics
-
Medical device
-
Others
-
-
Therapeutic Use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Oncology
-
CNS
-
Cardiovascular
-
Infectious disease
-
Metabolic disorders
-
Others
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
India
-
China
-
Japan
-
South Korea
-
Australia
-
Thailand
-
Singapore
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global clinical trial supplies market size was estimated at USD 2.07 billion in 2022 and is expected to reach USD 2.2 billion in 2023.
b. The global clinical trial supplies market is expected to grow at a compound annual growth rate of 6.5% from 2023 to 2030 to reach USD 3.40 billion by 2030.
b. North America dominated the clinical trial supplies market with a share of 55.49% in 2022. This is attributable to the high share of clinical trials conducted in this region and a greater number of clinical trials supply players with the most advanced technology.
b. Some key players operating in the clinical trial supplies market include KLIFO A/S, PAREXEL International Corporation, Almac Group Ltd, Movianto GmbH, Biocair International Ltd., PCI Pharma Services, and Thermo Fischer Scientific.
b. Key factors that are driving the clinical trial supplies market growth include globalization and a rise in the number of clinical trials, increasing complexities, and a rising number of biologics and biosimilar drugs in trials.
Table of Contents
Chapter 1. Methodology and Scope
1.1. Market Segmentation and Scope
1.2. Market Definitions
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased Database
1.4.2. GVR’s Internal Database
1.5. Details of primary research
1.5.1. Data for primary interviews in North America
1.5.2. Data for primary interviews in Europe
1.5.3. Data for primary interviews in Asia Pacific
1.5.4. Data for primary interviews in Latin America
1.5.5. Data for Primary interviews in MEA
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.1.1. Approach 1: Commodity flow approach
1.7.2. Volume price analysis (Model 2)
1.7.2.1. Approach 2: Volume price analysis
1.8. Research Scope and Assumptions
1.8.1. List of Secondary Sources
1.8.2. List of Primary Sources
1.8.3. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.3. Competitive Insights
Chapter 3. Clinical Trial Supplies Market Variables, Trends, & Scope
3.1. Market Lineage Outlook
3.1.1. Parent Market Outlook
3.1.2. Related/Ancillary Market Outlook
3.2. Market Dynamics
3.2.1. Market Drivers Analysis
3.2.1.1. Expansion of clinical trials sites
3.2.1.2. Growing R&D in biologics and biosimilar
3.2.1.3. Advancements in supply chain technology
3.2.2. Market Restraints Analysis
3.2.2.1. Presence of stringent regulatory policies
3.2.2.2. High Possibility of Counterfeiting Drugs
3.3. Clinical Trial Supplies Market Analysis Tools
3.3.1. Porter’s Analysis
3.3.1.1. Bargaining power of the suppliers
3.3.1.2. Bargaining power of the buyers
3.3.1.3. Threats of substitution
3.3.1.4. Threats from new entrants
3.3.1.5. Competitive rivalry
3.3.2. PESTEL Analysis
3.3.2.1. Political landscape
3.3.2.2. Economic and Social landscape
3.3.2.3. Technological landscape
3.3.2.4. Environmental landscape
3.3.2.5. Legal landscape
Chapter 4. Clinical Trial Supplies Market: Source Estimates & Trend Analysis
4.1. Segment Dashboard
4.2. Clinical Trial Supplies: Source Movement Analysis, USD Billion, 2023 & 2030
4.3. Phase I
4.3.1. Phase I Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
4.4. Phase II
4.4.1. Phase II Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
4.5. Phase III
4.5.1. Phase III Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
4.6. Others
4.6.1. Others Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
Chapter 5. Clinical Trial Supplies Market: Product & Service Estimates & Trend Analysis
5.1. Segment Dashboard
5.2. Clinical Trial Supplies Market: Product & Service Movement Analysis, USD Billion, 2023 & 2030
5.3. Manufacturing
5.3.1. Manufacturing Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
5.3.2. Storage & Distribution
5.3.2.1. Downstream processing Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
5.3.2.2. Cold Chain Distribution
5.3.2.2.1. Cold Chain Distribution Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
5.3.2.3. Non-Cold Chain Distribution
5.3.2.3.1. Non-Cold Chain Distribution Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
5.3.3. Supply Chain Management
5.3.3.1. Supply Chain Management Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
5.4. Fill & finish operations
5.4.1. Fill & finish operations Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
5.5. Analytical & QC studies
5.5.1. Analytical & QC studies Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
5.6. Packaging
5.6.1. Packaging Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
Chapter 6. Clinical Trial Supplies Market: Therapeutic Area Estimates & Trend Analysis
6.1. Segment Dashboard
6.2. Clinical Trial Supplies Market: Therapeutic Area Movement Analysis, USD Billion, 2023 & 2030
6.3. Biologics
6.3.1. Biologics Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
6.3.2. Oncology
6.3.2.1. Oncology Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
6.3.3. CNS
6.3.4. CNS Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
6.3.5. Cardiovascular
6.3.5.1. Cardiovascular Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
6.3.6. Infectious disease
6.3.6.1. Infectious disease Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
6.3.7. Metabolic disorders
6.3.7.1. Metabolic disorders Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
6.4. Others
6.4.1. Others Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
Chapter 7. Clinical Trial Supplies Market: End Use Estimates & Trend Analysis
7.1. Segment Dashboard
7.2. Clinical Trial Supplies Market: End Use Movement Analysis, USD Billion, 2023 & 2030
7.3. Pharmaceutical
7.3.1. Pharmaceutical Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
7.3.2. Biologics
7.3.2.1. Oncology Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
7.3.3. Medical Devices
7.3.3.1. Medical Devices Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
7.3.4. Others
7.3.4.1. Others Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Billion)
Chapter 8. Clinical Trial Supplies Market: Regional Estimates & Trend Analysis
8.1. Clinical Trial Supplies Market Share, By Region, 2023 & 2030, USD Billion
8.2. North America
8.2.1. North America Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.2.2. U.S.
8.2.2.1. Key Country Dynamics
8.2.2.2. Regulatory Landscape/Reimbursement Scenario
8.2.2.3. Competitive Insights
8.2.2.4. U.S. Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.2.3. Canada
8.2.3.1. Key Country Dynamics
8.2.3.2. Regulatory Landscape/Reimbursement Scenario
8.2.3.3. Competitive Insights
8.2.3.4. Canada Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.3. Europe
8.3.1. Europe Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.3.2. U.K.
8.3.2.1. Key Country Dynamics
8.3.2.2. Regulatory Landscape/Reimbursement Scenario
8.3.2.3. Competitive Insights
8.3.2.4. U.K. Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.3.3. Germany
8.3.3.1. Key Country Dynamics
8.3.3.2. Regulatory Landscape/Reimbursement Scenario
8.3.3.3. Competitive Insights
8.3.3.4. Germany Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.3.4. France
8.3.4.1. Key Country Dynamics
8.3.4.2. Regulatory Landscape/Reimbursement Scenario
8.3.4.3. Competitive Insights
8.3.4.4. France Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.3.5. Italy
8.3.5.1. Key Country Dynamics
8.3.5.2. Regulatory Landscape/Reimbursement Scenario
8.3.5.3. Competitive Insights
8.3.5.4. Italy Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.3.6. Spain
8.3.6.1. Key Country Dynamics
8.3.6.2. Regulatory Landscape/Reimbursement Scenario
8.3.6.3. Competitive Insights
8.3.6.4. Spain Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.3.7. Sweden
8.3.7.1. Key Country Dynamics
8.3.7.2. Regulatory Landscape/Reimbursement Scenario
8.3.7.3. Competitive Insights
8.3.7.4. Sweden Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.3.8. Denmark
8.3.8.1. Key Country Dynamics
8.3.8.2. Regulatory Landscape/Reimbursement Scenario
8.3.8.3. Competitive Insights
8.3.8.4. Denmark Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.3.9. Norway
8.3.9.1. Key Country Dynamics
8.3.9.2. Regulatory Landscape/Reimbursement Scenario
8.3.9.3. Competitive Insights
8.3.9.4. Norway Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.4. Asia Pacific
8.4.1. Asia Pacific Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.4.2. China
8.4.2.1. Key Country Dynamics
8.4.2.2. Regulatory Landscape/Reimbursement Scenario
8.4.2.3. Competitive Insights
8.4.2.4. China Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.4.3. Japan
8.4.3.1. Key Country Dynamics
8.4.3.2. Regulatory Landscape/Reimbursement Scenario
8.4.3.3. Competitive Insights
8.4.3.4. Japan Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.4.4. India
8.4.4.1. Key Country Dynamics
8.4.4.2. Regulatory Landscape/Reimbursement Scenario
8.4.4.3. Competitive Insights
8.4.4.4. India Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.4.5. South Korea
8.4.5.1. Key Country Dynamics
8.4.5.2. Regulatory Landscape/Reimbursement Scenario
8.4.5.3. Competitive Insights
8.4.5.4. South Korea Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.4.6. Australia
8.4.6.1. Key Country Dynamics
8.4.6.2. Regulatory Landscape/Reimbursement Scenario
8.4.6.3. Competitive Insights
8.4.6.4. Australia Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.4.7. Thailand
8.4.7.1. Key Country Dynamics
8.4.7.2. Regulatory Landscape/Reimbursement Scenario
8.4.7.3. Competitive Insights
8.4.7.4. Thailand Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.4.8. Singapore
8.4.8.1. Key Country Dynamics
8.4.8.2. Regulatory Landscape/Reimbursement Scenario
8.4.8.3. Competitive Insights
8.4.8.4. Singapore Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.5. Latin America
8.5.1. Latin America Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.5.2. Brazil
8.5.2.1. Key Country Dynamics
8.5.2.2. Regulatory Landscape/Reimbursement Scenario
8.5.2.3. Competitive Insights
8.5.2.4. Brazil Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.5.3. Mexico
8.5.3.1. Key Country Dynamics
8.5.3.2. Regulatory Landscape/Reimbursement Scenario
8.5.3.3. Competitive Insights
8.5.3.4. Mexico Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.5.4. Argentina
8.5.4.1. Key Country Dynamics
8.5.4.2. Regulatory Landscape/Reimbursement Scenario
8.5.4.3. Competitive Insights
8.5.4.4. Argentina Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.6. Middle East and Africa
8.6.1. Middle East and Africa Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.6.2. Saudi Arabia
8.6.2.1. Key Country Dynamics
8.6.2.2. Regulatory Landscape/Reimbursement Scenario
8.6.2.3. Competitive Insights
8.6.2.4. Saudi Arabia Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.6.3. UAE
8.6.3.1. Key Country Dynamics
8.6.3.2. Regulatory Landscape/Reimbursement Scenario
8.6.3.3. Competitive Insights
8.6.3.4. UAE Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.6.4. South Africa
8.6.4.1. Key Country Dynamics
8.6.4.2. Regulatory Landscape/Reimbursement Scenario
8.6.4.3. Competitive Insights
8.6.4.4. South Africa Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
8.6.5. Kuwait
8.6.5.1. Key Country Dynamics
8.6.5.2. Regulatory Landscape/Reimbursement Scenario
8.6.5.3. Competitive Insights
8.6.5.4. Kuwait Clinical Trial Supplies Market Estimates and Forecasts, 2018 - 2030 (USD Billion)
Chapter 9. Competitive Landscape
9.1. Recent Developments & Impact Analysis by Key Market Participants
9.2. Company Categorization
9.3. Company Market Share Analysis
9.4. Company Heat Map Analysis
9.5. Strategy Mapping
9.5.1. Expansion
9.5.2. Mergers & Acquisition
9.5.3. Partnerships & Collaborations
9.5.4. New Product Launches
9.5.5. Research And Development
9.6. Company Profiles
9.6.1. Almac Group
9.6.1.1. Participant’s Overview
9.6.1.2. Financial Performance
9.6.1.3. Product Benchmarking
9.6.1.4. Recent Developments
9.6.2. Biocair
9.6.2.1. Participant’s Overview
9.6.2.2. Financial Performance
9.6.2.3. Product Benchmarking
9.6.2.4. Recent Developments
9.6.3. Catalent, Inc.
9.6.3.1. Participant’s Overview
9.6.3.2. Financial Performance
9.6.3.3. Product Benchmarking
9.6.3.4. Recent Developments
9.6.4. KLIFO
9.6.4.1. Participant’s Overview
9.6.4.2. Financial Performance
9.6.4.3. Product Benchmarking
9.6.4.4. Recent Developments
9.6.5. Movianto
9.6.5.1. Participant’s Overview
9.6.5.2. Financial Performance
9.6.5.3. Product Benchmarking
9.6.5.4. Recent Developments
9.6.6. PCI Pharma Services
9.6.6.1. Participant’s Overview
9.6.6.2. Financial Performance
9.6.6.3. Product Benchmarking
9.6.6.4. Recent Developments
9.6.7. Sharp Services, LLC
9.6.7.1. Participant’s Overview
9.6.7.2. Financial Performance
9.6.7.3. Product Benchmarking
9.6.7.4. Recent Developments
9.6.8. Thermo Fisher Scientific, Inc.
9.6.8.1. Participant’s Overview
9.6.8.2. Financial Performance
9.6.8.3. Product Benchmarking
9.6.8.4. Recent Developments
9.6.9. Marken
9.6.9.1. Participant’s Overview
9.6.9.2. Financial Performance
9.6.9.3. Product Benchmarking
9.6.9.4. Recent Developments
9.6.10. Paraxel International Corporation
9.6.10.1. Participant’s Overview
9.6.10.2. Financial Performance
9.6.10.3. Product Benchmarking
9.6.10.4. Recent Developments
List of Tables
Table 1 List of Abbreviations
Table 2 North America clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 3 North America clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 4 North America clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 5 North America clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 6 US clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 7 US clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 8 US clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 9 US clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 10 Canada clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 11 Canada clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 12 Canada clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 13 Canada clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 14 Europe clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 15 Europe clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 16 Europe clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 17 Europe clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 18 UK clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 19 UK clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 20 UK clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 21 UK clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 22 Germany clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 23 Germany clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 24 Germany clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 25 Germany clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 26 France clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 27 France clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 28 France clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 29 France clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 30 Italy clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 31 Italy clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 32 Italy clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 33 Italy clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 34 Spain clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 35 Spain clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 36 Spain clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 37 Spain clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 38 Denmark clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 39 Denmark clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 40 Denmark clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 41 Denmark clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 42 Sweden clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 43 Sweden clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 44 Sweden clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 45 Sweden clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 46 Norway clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 47 Norway clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 48 Norway clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 49 Norway clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 50 Asia Pacific clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 51 Asia Pacific clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 52 Asia Pacific clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 53 Asia Pacific clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 54 Japan clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 55 Japan clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 56 Japan clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 57 Japan clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 58 China clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 59 China clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 60 China clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 61 China clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 62 India clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 63 India clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 64 India clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 65 India clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 66 Australia clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 67 Australia clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 68 Australia clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 69 Australia clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 70 South Korea clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 71 South Korea clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 72 South Korea clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 73 South Korea clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 74 Thailand clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 75 Thailand clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 76 Thailand clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 77 Thailand clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 78 Latin America clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 79 Latin America clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 80 Latin America clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 81 Latin America clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 82 Brazil clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 83 Brazil clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 84 Brazil clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 85 Brazil clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 86 Mexico clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 87 Mexico clinical trials supply chain management market, by type, 2018 - 2030 (USD Billion)
Table 88 Mexico clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 89 Mexico clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 90 MEA clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 91 MEA clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 92 MEA clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 93 MEA clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 94 South Africa clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 95 South Africa clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 96 South Africa clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 97 South Africa clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 98 Saudi Arabia clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 99 Saudi Arabia clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 100 Saudi Arabia clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 101 Saudi Arabia clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 102 UAE clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 103 UAE clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 104 UAE clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 105 UAE clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
Table 106 Kuwait clinical trial supplies market, by product and services, 2018 - 2030 (USD Billion)
Table 107 Kuwait clinical trial supplies market, by end-use, 2018 - 2030 (USD Billion)
Table 108 Kuwait clinical trial supplies market, by therapeutics use, 2018 - 2030 (USD Billion)
Table 109 Kuwait clinical trial supplies market, by clinical phases, 2018 - 2030 (USD Billion)
List of Figures
Fig. 1 Market research process
Fig. 2 Information procurement
Fig. 3 Primary research pattern
Fig. 4 Market research approaches
Fig. 5 Value chain-based sizing & forecasting
Fig. 6 QFD modeling for market share assessment
Fig. 7 Market summary (Figures in USD Billion, 2022)
Fig. 8 Market trends & outlook
Fig. 9 Clinical trial supplies market: Market segmentation
Fig. 10 Clinical trial supplies market revenue, (USD Billion), 2018 - 2030
Fig. 11 Clinical trial supplies: Porter’s analysis
Fig. 12 Clinical trial supplies: PESTEL analysis
Fig. 13 Clinical trial supplies market phase outlook key takeaways
Fig. 14 Clinical trial supplies market: Phase movement analysis
Fig. 15 Phase I market, 2018 - 2030 (USD Billion)
Fig. 16 Phase II market, 2018 - 2030 (USD Billion)
Fig. 17 Phase III market, 2018 - 2030 (USD Billion)
Fig. 18 Others market, 2018 - 2030 (USD Billion)
Fig. 19 Clinical trial supplies market product/service outlook key takeaways
Fig. 20 Clinical trial supplies market: Product/Service movement analysis
Fig. 21 Manufacturing market, 2018 - 2030 (USD Billion)
Fig. 22 Storage & distribution market, 2018 - 2030 (USD Billion)
Fig. 23 Cold chain-based storage & distribution market, 2018 - 2030 (USD Billion)
Fig. 24 Non-cold chain-based storage & distribution market, 2018 - 2030 (USD Billion)
Fig. 25 Supply chain management market, 2018 - 2030 (USD Billion)
Fig. 26 Clinical trial supplies market end-use outlook key takeaways
Fig. 27 Clinical trial supplies market: End-use movement analysis
Fig. 28 Pharmaceuticals market, 2018 - 2030 (USD Billion)
Fig. 29 Biologics market, 2018 - 2030 (USD Billion)
Fig. 30 Medical device market, 2018 - 2030 (USD Billion)
Fig. 31 Other end-use market, 2018 - 2030 (USD Billion)
Fig. 32 Clinical trial supplies market therapeutic use outlook key takeaways
Fig. 33 Clinical trial supplies market: Therapeutic Use movement analysis
Fig. 34 Oncology market, 2018 - 2030 (USD Billion)
Fig. 35 CNS market, 2018 - 2030 (USD Billion)
Fig. 36 Cardiovascular market, 2018 - 2030 (USD Billion)
Fig. 37 Infectious disease market, 2018 - 2030 (USD Billion)
Fig. 38 Metabolic disorders market, 2018 - 2030 (USD Billion)
Fig. 39 Other therapeutic use market, 2018 - 2030 (USD Billion)
Fig. 40 Regional marketplace: Key takeaways
Fig. 41 North America clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 42 U.S. clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 43 Canada clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 44 Europe clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 45 UK clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 46 Germany clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 47 France clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 48 Italy clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 49 Spain clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 50 Denmark clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 51 Sweden clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 52 Norway clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 53 Asia Pacific clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 54 Japan clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 55 China clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 56 India clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 57 Australia clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 58 South Korea clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 59 Thailand clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 60 Latin America clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 61 Brazil clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 62 Mexico clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 63 Argentina clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 64 MEA clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 65 South Africa clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 66 Saudi Arabia clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 67 UAE clinical trial supplies market, 2018 - 2030 (USD Billion)
Fig. 68 Kuwait clinical trial supplies market, 2018 - 2030 (USD Billion)What questions do you have? Get quick response from our industry experts. Request a Free ConsultationMarket Segmentation
- Clinical Trial Supplies Clinical Phase Outlook (Revenue, USD Billion, 2018 - 2030)
- Phase I
- Phase II
- Phase III
- Others
- Clinical Trial Supplies Product/Service Outlook (Revenue, USD Billion, 2018 - 2030)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Clinical Trial Supplies End-Use Outlook (Revenue, USD Billion, 2018 - 2030)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Clinical Trial Supplies Therapeutic Use Outlook (Revenue, USD Billion, 2018 - 2030)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Clinical Trial Supplies Market: Regional Outlook (Revenue, USD Billion, 2018- 2030)
- North America
- North America Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- North America Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- North America Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- North America Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- U.S.
- U.S. Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- U.S. Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- U.S. Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- U.S. Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- U.S. Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Canada
- Canada Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Canada Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Canada Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Canada Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Canada Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- North America Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Europe
- Europe Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Europe Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Europe Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Europe Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- UK
- UK Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- UK Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- UK Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- UK Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- UK Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Germany
- Germany Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Germany Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Germany Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Germany Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Germany Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- France
- France Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- France Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- France Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- France Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- France Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Italy
- Italy Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Italy Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Italy Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Italy Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Italy Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Spain
- Spain Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Spain Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Spain Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Spain Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Spain Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Denmark
- Denmark Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Denmark Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Denmark Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Denmark Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Denmark Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Sweden
- Sweden Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Sweden Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Sweden Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Sweden Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Sweden Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Norway
- Norway Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Norway Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Norway Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Norway Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Norway Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Europe Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Asia Pacific
- Asia Pacific Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Asia Pacific Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Asia Pacific Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Asia Pacific Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- India
- India Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- India Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- India Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- India Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- India Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- China
- China Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- China Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- China Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- China Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- China Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Japan
- Japan Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Japan Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Japan Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Japan Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Japan Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- South Korea
- South Korea Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- South Korea Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- South Korea Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- South Korea Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- South Korea Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Australia
- Australia Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Australia Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Australia Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Australia Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Australia Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Thailand
- Thailand Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Thailand Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Thailand Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Thailand Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Thailand Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Singapore
- Singapore Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Singapore Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Singapore Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Singapore Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Singapore Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Asia Pacific Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Latin America
- Latin America Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Latin America Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Latin America Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Latin America Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Brazil
- Brazil Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Brazil Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Brazil Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Brazil Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Brazil Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Mexico
- Mexico Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Mexico Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Mexico Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Mexico Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Mexico Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Argentina
- Argentina Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Argentina Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Argentina Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Argentina Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Argentina Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Latin America Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Middle East and Africa (MEA)
- MEA Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- MEA Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- MEA Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- MEA Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- South Africa
- South Africa Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- South Africa Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- South Africa Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- South Africa Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- South Africa Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Saudi Arabia
- Saudi Arabia Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Saudi Arabia Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Saudi Arabia Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Saudi Arabia Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Saudi Arabia Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- UAE
- UAE Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- UAE Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- UAE Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- UAE Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- UAE Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Kuwait
- Kuwait Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Phase I
- Phase II
- Phase III
- Others
- Kuwait Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- Manufacturing
- Storage & distribution
- Cold chain based
- Non-cold chain based
- Supply chain management
- Kuwait Clinical Trial Supplies Market, By End-Use, 2018- 2030 (USD Billion)
- Pharmaceuticals
- Biologics
- Medical device
- Others
- Kuwait Clinical Trial Supplies Market, By Therapeutic Use, 2018- 2030 (USD Billion)
- Oncology
- CNS
- Cardiovascular
- Infectious disease
- Metabolic disorders
- Others
- Kuwait Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- MEA Clinical Trial Supplies Market, By Phase, 2018- 2030 (USD Billion)
- North America
Clinical Trial Supplies Market Dynamics
Driver: Clinical trial site expansions
Majority of clinical trials are currently being conducted in developing economies. Increasing costs of clinical trials and complications in patient recruitment have encouraged biopharmaceutical companies to outsource clinical trials to regions such as Asia Pacific, Latin America, Central & Eastern Europe, and the Middle East. Disease variation in developing economies further aids biopharmaceutical companies to perform clinical trials on rare diseases. Regions such as Asia Pacific also provide greater economic benefits to biopharmaceutical companies, as governments in Singapore and China allocate funds to promote biomedical research. In Latin America, patient recruitment is easy due to reduced language barriers, which can help obtain informed consent easily, resulting in faster clinical trial process.
Outsourcing clinical trials is increasing the demand for efficient supply chain logistics, which includes temperature control management and cold chain management. There has been a substantial growth in global biopharmaceutical spending on cold chain logistics, and this trend is expected to drive the clinical trial supplies market. As per a survey by Pharma-IQ in 2017, distribution, logistics, and manufacturing are the most outsourced clinical supply functions, with shares of 47.5%, 42.4%, & 37.4%, respectively. Therefore, outsourcing of clinical trials is expected to increase, which is expected to propel the demand for clinical trial supplies.
Driver: Rising R&D activities in biosimilars and biologics
There has been a significant rise in number of biologics and temperature-sensitive drugs in clinical trials. Currently, 38.0% of pharmaceutical drugs are temperature-sensitive and 35.0% of late-phase pharmaceutical drugs are biologics. This number is expected to increase in the future, as the demand for biologics is growing owing to lesser adverse effects as compared to traditional ties for storage of temperature-sensitive drugs. Hence, rise in number of biologics in clinical trials is expected to increase the demand for cold chain facilities. It has been estimated that eight out of top ten biopharmaceutical products will require cold chain facilities by 2022. The high demand for biosimilars in developing as well as developed economies is expected to further boost the cold chain supply, thereby contributing to the growth of clinical trial supplies market over the forecast period.
Restraint: Lack of proper reimbursement coverage
The biggest challenge to global clinical trial supplies market are the varying regional regulations and ethical dilemmas. Each country has its requirements with regard to shipments. In addition, each drug company has different storage requirements for testing drugs. For instance, FDA approval is required before any shipment carrying drugs can enter the U.S. The shipping and customs requirements of each country are also different, which makes it a complicated affair to conduct clinical trials in different countries. Recently, there has been a paradigm shift in clinical trials market, as most trials are now being conducted in developing economies, where researchers may not be properly trained or may be unaware of regulations governing clinical trials.
For instance, in Vietnam, shipments need to be addressed to organizations and not to individuals. If a shipment is not addressed properly, the individual may be held liable for duty and tax implications on the package. In certain countries, clearing customs may take more time depending on the number of shipments at a point in time. Due to the delay, the quality of the contents may deteriorate and affect the efficacy of clinical trials.
What Does This Report Include?
This section will provide insights into the contents included in this clinical trial supplies market report and help gain clarity on the structure of the report to assist readers in navigating smoothly.
Clinical trial supplies market qualitative analysis
-
Industry overview
-
Industry trends
-
Market drivers and restraints
-
Market size
-
Growth prospects
-
Porter’s analysis
-
PESTEL analysis
-
Key market opportunities prioritized
-
Competitive landscape
-
Company overview
-
Financial performance
-
Product benchmarking
-
Latest strategic developments
-
Clinical trial supplies market quantitative analysis
-
Market size, estimates, and forecast from 2018 to 2030
-
Market estimates and forecast for product segments up to 2030
-
Regional market size and forecast for product segments up to 2030
-
Market estimates and forecast for application segments up to 2030
-
Regional market size and forecast for application segments up to 2030
-
Company financial performance
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationResearch Methodology
A three-pronged approach was followed for deducing the clinical trial supplies market estimates and forecasts. The process has three steps: information procurement, analysis, and validation. The whole process is cyclical, and steps repeat until the estimates are validated. The three steps are explained in detail below:
Information procurement: Information procurement is one of the most extensive and important stages in our research process, and quality data is critical for accurate analysis. We followed a multi-channel data collection process for clinical trial supplies market to gather the most reliable and current information possible.
- We buy access to paid databases such as Hoover’s and Factiva for company financials, industry information, white papers, industry journals, SME journals, and more.
- We tap into Grand View’s proprietary database of data points and insights from active and archived monitoring and reporting.
- We conduct primary research with industry experts through questionnaires and one-on-one phone interviews.
- We pull from reliable secondary sources such as white papers and government statistics, published by organizations like WHO, NGOs, World Bank, etc., Key Opinion Leaders (KoL) publications, company filings, investor documents, and more.
- We purchase and review investor analyst reports, broker reports, academic commentary, government quotes, and wealth management publications for insightful third-party perspectives.
Analysis: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilized different methods of clinical trial supplies market data depending on the type of information we’re trying to uncover in our research.
-
Market Research Efforts: Bottom-up Approach for estimating and forecasting demand size and opportunity, top-down Approach for new product forecasting and penetration, and combined approach of both Bottom-up and Top-down for full coverage analysis.
-
Value-Chain-Based Sizing & Forecasting: Supply-side estimates for understanding potential revenue through competitive benchmarking, forecasting, and penetration modeling.
-
Demand-side estimates for identifying parent and ancillary markets, segment modeling, and heuristic forecasting.
-
Qualitative Functional Deployment (QFD) Modelling for market share assessment.
Market formulation and validation: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilize different methods of data analysis depending on the type of information we’re trying to uncover in our research.
-
Market Formulation: This step involves the finalization of market numbers. This step on an internal level is designed to manage outputs from the Data Analysis step.
-
Data Normalization: The final market estimates and forecasts are then aligned and sent to industry experts, in-panel quality control managers for validation.
-
This step also entails the finalization of the report scope and data representation pattern.
-
Validation: The process entails multiple levels of validation. All these steps run in parallel, and the study is forwarded for publishing only if all three levels render validated results.
Clinical Trial Supplies Market Categorization:
The clinical trial supplies market was categorized into five segments, namely clinical phase (Phase I, Phase II, Phase III), product & service (Manufacturing, Storage & Distribution, Supply chain management), end-use (Pharmaceutical, Biologics, Medical device), therapeutic use (Oncology, CNS, Cardiovascular, Infectious disease, Metabolic disorders), and region (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa).
Segment Market Methodology:
The clinical trial supplies market was segmented into clinical phase, product & service, end-use, therapeutic use, and regions. The demand at a segment level was deduced using a funnel method. Concepts like the TAM, SAM, SOM, etc., were put into practice to understand the demand. We at GVR deploy three methods to deduce market estimates and determine forecasts. These methods are explained below:
Market research approaches: Bottom-up
-
Demand estimation of each product across countries/regions summed up to from the total market.
-
Variable analysis for demand forecast.
-
Demand estimation via analyzing paid database, and company financials either via annual reports or paid database.
-
Primary interviews for data revalidation and insight collection.
Market research approaches: Top-down
-
Used extensively for new product forecasting or analyzing penetration levels.
-
Tool used invoice product flow and penetration models Use of regression multi-variant analysis for forecasting Involves extensive use of paid and public databases.
-
Primary interviews and vendor-based primary research for variable impact analysis.
Market research approaches: Combined
- This is the most common method. We apply concepts from both the top-down and bottom-up approaches to arrive at a viable conclusion.
Regional Market Methodology:
The clinical trial supplies market was analyzed at a regional level. The globe was divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, keeping in focus variables like consumption patterns, export-import regulations, consumer expectations, etc. These regions were further divided into twenty-three countries, namely, the U.S.; Canada; the UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; India; Japan; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa, Saudi Arabia; UAE; Kuwait.
All three above-mentioned market research methodologies were applied to arrive at regional-level conclusions. The regions were then summed up to form the global market.
Clinical trial supplies market companies & financials:
The clinical trial supplies market was analyzed via companies operating in the sector. Analyzing these companies and cross-referencing them to the demand equation helped us validate our assumptions and conclusions. Key market players analyzed include:
-
Almac Group is a contract development and manufacturing organization that specializes in providing integrated services, such as biomarker discovery, biomarker clinical trial management, companion diagnostic development, internal biomarker discovery, genomic services, proprietary discovery arrays, and bioinformatics consulting. It also offers physical, chemical, & analytical services, as well as clinical trial supply chain solutions such as manufacturing, packaging, labeling, binding, & distribution. Currently, Almac Clinical Services serves over 600 pharmaceutical and biotech firms globally, comprising 18 of the top 20 pharmaceutical companies. The company operates across 18 facilities, including Europe, the U.S., and Asia. It also has operations in the UK, Ireland, across the U.S., and in Asia (Singapore and Tokyo).
-
Biocair is a private company that specializes in logistics & courier services for biotechnological, life science, and pharmaceutical industries. Products offered by the company are regulatory compliance, global logistics, value-added services, customer-focused solutions, and temperature-controlled solutions. These products are mainly utilized by commercial, clinical trial, & preclinical industries, as well as pharmaceutical professionals & scientists. It is a global specialist in clinical trial supply chain management, providing personalized logistics solutions for domestic and international multisite clinical trials. The company has a presence in Europe, the U.S., Africa, and Asia. Its global network operates 24/7 in over 160 countries, providing GDP-compliant life sciences supply chain management solutions.
-
Catalent, Inc. is a leading global provider of advanced delivery technologies, development, and manufacturing solutions for drugs, biologics, cell & gene therapies, and consumer health products. The company currently operates in four segments: Softgel & Oral Technologies, Biologics, Oral & Specialty Delivery, and Clinical Supply Services. The Clinical Supply Services segment provides manufacturing, packaging, storage, distribution, and inventory management for drugs & biologics in clinical trials. The company has more than 180 products launched annually, and around 700 products are under development. It has accomplished more than 5,000 clinical trials with 150,000 shipments a year to more than 80 countries. As of June 30, 2020, the firm had 53 manufacturing facilities, of which, 22 facilities are in the U.S.
-
KLIFO is a Denmark-based company that provides consultancy services for clinical researchers, clinical trial supply, pharmacovigilance, regulatory affairs, and pharmaceutical & biotech products. Its services include feasibility & strategy studies, packaging, translation, labeling, preparation of protocols, and registration of pharmaceuticals. In addition, it offers database development, trial monitoring, clinical reports, and biostatistical analysis. The company completed 264 packaging campaigns and 2,200 shipments in 2019. The company operates in Denmark, Germany, Sweden, and the Netherlands.
-
Movianto is part of the Walden Group, a European leader in logistics and transports for pharma. It provides services for pharmaceutical, biotech, medical device, and diagnostic industries. It provides outsourcing services along the supply chain, such as warehousing, transportation, active temperature control logistics, and other value-added services. The company has over 275,000 pallet locations spread over a network of 20 wholly owned warehouses in 11 European countries. It mainly operates in 3 regions in Europe: West (the UK and Eire), North (Belgium, the Netherlands, Germany, Switzerland, Denmark, the Czech Republic and Slovakia), and South (France, Spain).
-
PCI Pharma Services, formerly known as Packaging Coordinators, Inc., provides clinical trial services, integrated pharmaceutical development, comprehensive commercial, and distribution services. In addition, it provides clinical packaging, primary packaging, clinical supplies management, and packaging design. These services are offered mainly to biotechnological and pharmaceutical industries. The company has operations in North America, Europe, and Asia Pacific.
-
Sharp is a global leader in advanced clinical supply chain services and contract pharmaceutical packaging with over 32 clinical depots globally. The company develops, designs, and manufactures packaging machinery, printed, poly mail, stretch sleeves, stock, side & horizontal load bags, retail display, and thermal imprint ribbons. In addition, it offers auto-bagging machines, soft goods packaging, and inline thermal transfer printing systems. The company has operating facilities in the U.S., the UK, Belgium, and the Netherlands. Sharp is a part of UDG Healthcare plc, a company providing commercial, clinical, and packaging services to the healthcare industry.
-
Thermo Fisher Scientific, Inc. provides analytical instruments, reagents & consumables, equipment, software, and services for manufacturing, analysis, research, and diagnostics under brands such as Thermo Scientific, Invitrogen, Unity Lab Services, Applied Biosystems, and Fisher Scientific. Thermo Scientific encompasses analytical instruments, lab equipment, and specialty diagnostics. Fisher Scientific offers enterprise solutions, instrument services, and provides parts & consumables in healthcare, safety, and science education. Applied Biosystems markets instruments and reagents for laboratory purposes. Invitrogen has products for genetic engineering, amplification, purification, quantification, and analysis. Thermo Fisher Scientific offers pharma services solutions for drug development, clinical trial logistics, and commercial manufacturing to customers through its Patheon brand. Patheon offers integrated, end-to-end capabilities across all phases of development, including API, biologics, viral vectors, cGMP plasmids, formulation, clinical trials solutions, logistics services, and commercial manufacturing & packaging.
-
Marken is a fully owned subsidiary of UPS. The UPS Healthcare division employs 5,500 people in 128 sites across the world, including Polar Speed and Marken. For clinical drug product storage and distribution, the company provides GMP-compliant depot network and logistic hubs in 58 locations globally. It also offers biological sample shipments, Direct-to-Patient & home healthcare services, and biological kit manufacturing. It manages over 110,000 drug products and biological sample consignments monthly at all temperature ranges in around 220 countries. Additional services include storage, and distribution, supplementary material sourcing and GDP, regulatory, & compliance consulting.
-
PAREXEL International Corporation offers services such as medical communications, clinical research, advanced technology products & services, consulting, and commercialization. These services are utilized by biotechnological, pharmaceutical, and medical device companies. The company operates in three segments: PAREXEL Consulting Services, PAREXEL Informatics, and Clinical Research Services. Services offered by the CRS segment include biostatistics, clinical trial management, observational studies, clinical logistics, and patient disease registries.
Value chain-based sizing & forecasting
Supply Side Estimates
-
Company revenue estimation via referring to annual reports, investor presentations, and Hoover’s.
-
Segment revenue determination via variable analysis and penetration modeling.
-
Competitive benchmarking to identify market leaders and their collective revenue shares.
-
Forecasting via analyzing commercialization rates, pipelines, market initiatives, distribution networks, etc.
Demand side estimates
-
Identifying parent markets and ancillary markets
-
Segment penetration analysis to obtain pertinent
-
revenue/volume
-
Heuristic forecasting with the help of subject matter experts
-
Forecasting via variable analysis
Clinical Trial Supplies Market Report Objectives:
-
Understanding market dynamics (in terms of drivers, restraints, & opportunities) in the countries.
-
Understanding trends & variables in the individual countries & their impact on growth and using analytical tools to provide high-level insights into the market dynamics and the associated growth pattern.
-
Understanding market estimates and forecasts (with the base year as 2022, historic information from 2018 to 2021, and forecast from 2023 to 2030). Regional estimates & forecasts for each category are available and are summed up to form the global market estimates.
Clinical Trial Supplies Market Report Assumptions:
-
The report provides market value for the base year 2022 and a yearly forecast till 2030 in terms of revenue/volume or both. The market for each of the segment outlooks has been provided on region & country basis for the above-mentioned forecast period.
-
The key industry dynamics, major technological trends, and application markets are evaluated to understand their impact on the demand for the forecast period. The growth rates were estimated using correlation, regression, and time-series analysis.
-
We have used the bottom-up approach for market sizing, analyzing key regional markets, dynamics, & trends for various products and end-users. The total market has been estimated by integrating the country markets.
-
All market estimates and forecasts have been validated through primary interviews with the key industry participants.
-
Inflation has not been accounted for to estimate and forecast the market.
-
Numbers may not add up due to rounding off.
-
Europe consists of EU-8, Central & Eastern Europe, along with the Commonwealth of Independent States (CIS).
-
Asia Pacific includes South Asia, East Asia, Southeast Asia, and Oceania (Australia & New Zealand).
-
Latin America includes Central American countries and the South American continent
-
Middle East includes Western Asia (as assigned by the UN Statistics Division) and the African continent.
Primary Research
GVR strives to procure the latest and unique information for reports directly from industry experts, which gives it a competitive edge. Quality is of utmost importance to us, therefore every year we focus on increasing our experts’ panel. Primary interviews are one of the critical steps in identifying recent market trends and scenarios. This process enables us to justify and validate our market estimates and forecasts to our clients. With more than 8,000 reports in our database, we have connected with some key opinion leaders across various domains, including healthcare, technology, consumer goods, and the chemical sector. Our process starts with identifying the right platform for a particular type of report, i.e., emails, LinkedIn, seminars, or telephonic conversation, as every report is unique and requires a differentiated approach.
We send out questionnaires to different experts from various regions/ countries, which is dependent on the following factors:
-
Report/Market scope: If the market study is global, we send questionnaires to industry experts across various regions, including North America, Europe, Asia Pacific, Latin America, and MEA.
-
Market Penetration: If the market is driven by technological advancements, population density, disease prevalence, or other factors, we identify experts and send out questionnaires based on region or country dominance.
The time to start receiving responses from industry experts varies based on how niche or well-penetrated the market is. Our reports include a detailed chapter on the KoL opinion section, which helps our clients understand the perspective of experts already in the market space.
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationShare this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





