- Home
- »
- Pharmaceuticals
- »
-
Asia Pacific Radiopharmaceuticals Market Size Report, 2030GVR Report cover
![Asia Pacific Radiopharmaceuticals Market Report]()
Asia Pacific Radiopharmaceuticals Market Analysis By Product (Diagnostics, Therapeutics), By Type (Catheter, Doppler OCT, Handheld, Tabletop), By Application, By End Use, By Region, And Segment Forecasts 2024 - 2030
- Report ID: GVR-1-68038-172-6
- Number of Report Pages: 90
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Report Overview
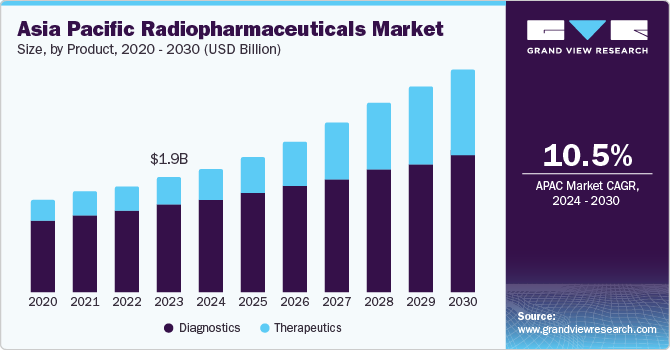
The Asia Pacific radiopharmaceuticals market size was valued at USD 1.91 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 10.5% from 2024 to 2030. The increasing prevalence of chronic diseases such as cancer and cardiovascular conditions has heightened the demand for advanced diagnostic and therapeutic solutions. Radiopharmaceuticals, which are crucial in nuclear medicine for imaging and treatment, are becoming more widely adopted due to their effectiveness.
Advancements in healthcare infrastructure and technology, including the development of PET/CT and SPECT systems, are facilitating better diagnostic capabilities. Government support and favorable policies are also playing a significant role in promoting the use of radiopharmaceuticals. Furthermore, rising healthcare expenditures and growing awareness about early disease detection are contributing to the market’s expansion.
Product Insights & Trends
Diagnostic products dominated the market and accounted for a share of 75.5% in 2023 driven by the rising prevalence of chronic diseases such as cancer and cardiovascular conditions, which require advanced diagnostic tools for early detection. Technological advancements in imaging techniques, such as PET and SPECT, have enhanced diagnostic accuracy and efficiency. Increased awareness among healthcare professionals and patients about the benefits of early diagnosis and the expansion of healthcare infrastructure and diagnostic centers further boost the adoption of radiopharmaceuticals.
The therapeutics segment is projected to grow at a CAGR of 18.6% from 2024 to 2030 attributed to ongoing advancements in nuclear medicine technology, which are enhancing the efficacy and safety of therapeutic radiopharmaceuticals. The growing focus on personalized medicine, which tailors treatments to individual patient profiles, is also boosting the adoption of these therapies. Furthermore, supportive government policies and increased funding for research and development in the healthcare sector are facilitating the growth of this segment.
Application Insights & Trends
The urology segment held the largest market share in 2023 attributed to the high prevalence of urological disorders, including prostate cancer, which is one of the most common cancers among men in the region. Radiopharmaceuticals are extensively used in the diagnosis and treatment of prostate cancer, particularly through techniques such as PET/CT scans and targeted radionuclide therapy.
The endocrine tumor segment is expected to grow significantly over the forecast period, driven by the rising incidence of endocrine-related cancers and advancements in nuclear medicine. The development of novel radiopharmaceuticals that target specific endocrine tumor markers has improved the accuracy of diagnosis and the efficacy of treatments. Moreover, the increasing focus on personalized medicine and targeted therapies is propelling the growth of this segment.
End Use Insights & Trends
The hospital segment dominated the market in 2023 attributed to the availability of competent medical practitioners in hospital radiology departments. Also, governments and organizations are providing funding and support for the development and commercialization of radiopharmaceuticals, driving the market growth. The hospital segment is expected to hold a considerable share of the market during the forecast period.
The diagnostic centers segment is expected to grow at a CAGR of 7.3% from 2024 to 2030 driven by the increasing demand for specialized diagnostic services and the rising prevalence of chronic diseases that require advanced imaging techniques for accurate diagnosis. Diagnostic centers are becoming more prevalent due to their ability to offer focused and efficient diagnostic services, often with shorter wait times compared to hospitals. The adoption of cutting-edge imaging technologies in these centers, coupled with the growing awareness of the benefits of early disease detection, is propelling the demand for radiopharmaceuticals. Furthermore, the expansion of diagnostic centers in urban and rural areas makes advanced diagnostic services more accessible to a broader population, thereby contributing to the segment’s anticipated growth. The emphasis on preventive healthcare and regular health check-ups is also encouraging the use of radiopharmaceuticals in diagnostic centers, supporting their significant market expansion.
Country Insights & Trends
Japan dominated the market in 2023 attributed to extensive research and development efforts in the pharmaceutical sector. The government's focus on education and research, particularly in healthcare technology and services, has contributed to this advancement. In Japan, significant investments are being made in radiopharmaceutical research and development, including the establishment of specialized research institutes and centers, as well as funding for AI-related projects.
India radiopharmaceuticals market is projected to grow at a CAGR of 12.5% over the forecast period. This growth is being driven by factors such as a large population, supportive government policies, a high prevalence of chronic diseases, and the increasing trend of medical tourism.
China radiopharmaceuticals market is expected to demonstrate robust expansion, reflecting its ever-growing influence in the global healthcare and pharmaceutical sectors. This growth can be attributed largely to its massive population base, which creates significant demand. China's government has been proactively supporting the sector through favorable policies, which have not only bolstered domestic capabilities but also attracted foreign investment into the sector, further propelling growth.
Key Asia Pacific Radiopharmaceuticals Company Share & Insights
The Asia Pacific radiopharmaceuticals market is driven by major companies such as Nordion, NTP, Bracco Group, and Mallinckrodt, among others.
-
Bracco Group offers high-quality imaging agents and diagnostic solutions. The company’s products are widely used in medical imaging to enhance the clarity and accuracy of diagnostic scans.
-
Mallinckrodt’s radiopharmaceuticals are employed in various diagnostic and therapeutic procedures, including cancer treatment and cardiovascular imaging. The company’s strong distribution network and emphasis on quality and safety contribute to its prominent market position.
Key Asia Pacific Radiopharmaceuticals Companies:
- Nordion Inc.
- Bracco
- IRE - IRE ELiT - BE
- NTP
- ANSTO
- ECZACIBAŞI-MONROL
- Lantheus
- Eckert & Ziegler
- Mallinckrodt company
- Cardinal Health.
Recent Developments
-
In July 2024, Full-Life Technologies partnered with SK Biopharmaceuticals to advance its FL-091 radiopharmaceutical compound into a global anti-cancer therapy. The companies signed a USD 571.5 million licensing agreement granting SK Biopharmaceuticals exclusive worldwide rights to develop and commercialize the innovative treatment.
-
In May 2024, Novartis announced a significant expansion of its collaboration with PeptiDream, investing USD 180 million upfront to boost its radioligand therapy (RLT) portfolio. The companies will jointly develop both therapeutic and diagnostic RLT products using PeptiDream's proprietary Peptide Discovery Platform System. This technology, previously employed for peptide-drug conjugates, will now be applied to identify and optimize macrocyclic peptides for conjugation to radionuclides against targets selected by Novartis.
Asia Pacific Radiopharmaceuticals Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 2.07 billion
Revenue forecast in 2030
USD 3.76 billion
Growth Rate
CAGR of 10.5% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Country scope
Japan, China, India, Australia, South Korea, Singapore, New Zealand, Philippines, Thailand
Key companies profiled
Nordion Inc., Bracco, IRE - IRE ELiT - BE, NTP, ANSTO, ECZACIBAŞI-MONROL, Lantheus, Eckert & Ziegler, Mallinckrodt company, Cardinal Health
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Asia Pacific Radiopharmaceuticals Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global artificial intelligence market report based on product, application, end use, and region.
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Diagnostics
-
SPECT
-
PET
-
-
Therapeutics
-
Alpha Emitters
-
Beta Emitters
-
Brachytherapy
-
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Cardiology
-
SPECT
-
PET
-
-
Neurology
-
Oncology
-
Thyroid
-
SPECT
-
Therapeutic Applications
-
-
Lymphoma
-
Bone Metastasis
-
SPECT
-
Therapeutic Applications
-
-
Endocrine Tumor
-
Pulmonary Scans
-
Urology
-
Others
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Diagnostic Centers
-
-
Country Outlook (Revenue, USD Million, 2018 - 2030)
-
Asia Pacific
-
Japan
-
China
-
India
-
Singapore
-
Australia
-
South Korea
-
New Zealand
-
Thailand
-
Philippines
-
-
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





