Asthma Spacers Market Size & Trends
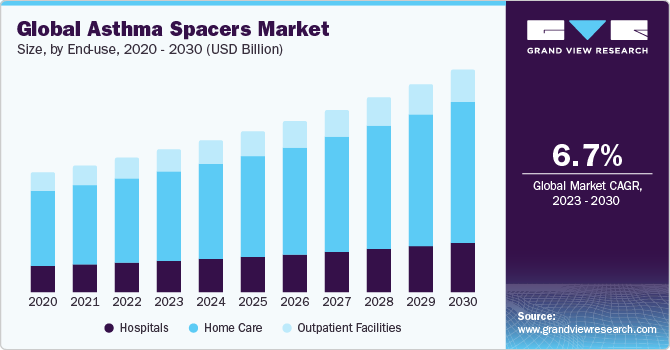
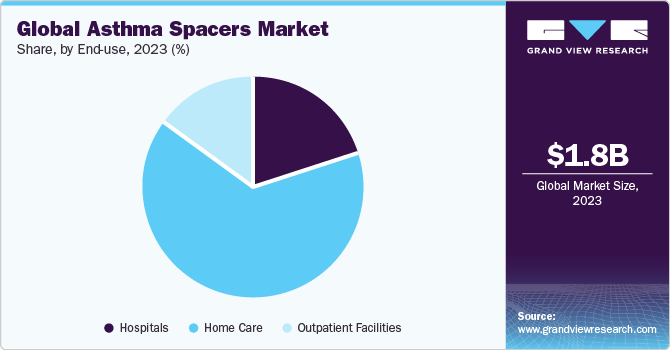
The global asthma spacers market was valued at USD 1.8 billion in 2023 and is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.7% from 2024 to 2030.. Effective management of asthma often requires the use of inhalers, which deliver medication directly to the lungs. Asthma spacers, also known as inhaler spacers or valved holding chambers, have emerged as crucial accessories in asthma management. They help optimize the medication delivery, ensuring a higher percentage reaches the lungs. The global asthma spacers market has witnessed significant growth in recent years, driven by innovative product developments and the involvement of leading companies.
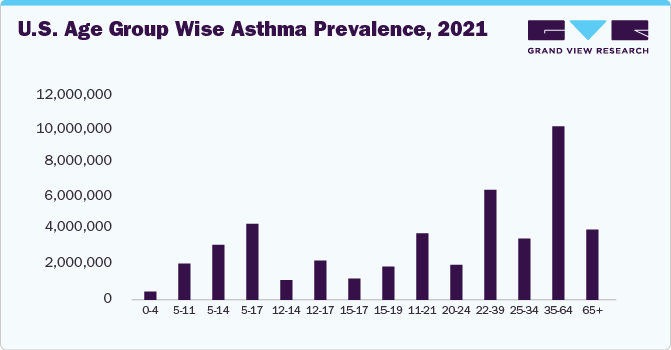
The rising prevalence of asthma, particularly among children, has fueled the demand for asthma spacers. With asthma affecting more than 300 million people worldwide, the market is poised for consistent growth. In the U.S., around 24 million people were diagnosed with asthma in 2021, with more than 80.0% of adults suffering from the same.
Increased awareness about the importance of proper inhaler technique and the role of asthma spacers in medication delivery has driven their adoption. Healthcare providers are increasingly educating patients on the benefits of using spacers. Moreover, the asthma spacer market has witnessed multiple advancements in design and materials, making them more user-friendly and effective. These innovations have led to improved patient compliance and medication adherence. Pediatric asthma spacers have gained traction as they cater to the specific needs of children. They come in colorful designs and are easy to use, making them an attractive option for young asthma patients.
Asthma is an increasingly common respiratory condition affecting millions of people worldwide. Its prevalence varies by region and is more commonly reported in developed countries and urban areas. The causes of asthma are complex, influenced by both genetic and environmental factors such as air pollution and allergen exposure. While the exact cause remains unknown, understanding the contributing factors and associated statistics is crucial in developing effective prevention and management strategies. Given the increasing prevalence of asthma, efforts to improve asthma control and reduce the burden of this condition remain a global public health priority. Inhalers are commonly used to manage asthma, but asthma spacers have emerged as essential accessories to optimize their effectiveness. These spacers help ensure that more medication reaches the lungs, leading to improved asthma management. The global market for asthma spacers has witnessed significant growth in recent years due to evolving market dynamics and innovative product developments by leading companies.
Type Insights
The asthma spacers market is segmented based on the type: Volumatic, Aerochamber, InspirEase, and Optichamber. Volumatic is a popular type of valved holding chamber (VHC) that is specifically designed to be used with metered-dose inhalers (MDIs). It is an excellent accessory for individuals with asthma, as it helps improve medication delivery to the lungs.
The unique feature of Volumatic is that it reduces the need for precise timing during inhalation, making it easier for the user to coordinate the inhaler actuation with their breathing. Due to its ease of use, the Volumatic asthma spacer is expected to dominate the global asthma spacer market during the forecast period.
End-use Insights
Based on end-use, the asthma spacers market is segmented into hospitals, home care, and outpatient facilities. The home care segment dominated the market in 2023. Asthma spacers, also called aerosol-holding chambers or inhaler spacers, are medical devices designed to improve medication delivery from an inhaler (metered-dose inhaler or MDI) to the lungs. These spacers are particularly beneficial for people who may find it difficult to coordinate the inhalation process with the activation of the inhaler, such as children and the elderly.
However, before using an asthma spacer at home, it is essential to consult with a healthcare professional, such as a doctor or a respiratory therapist. The healthcare professional will prescribe the appropriate type of spacer and medication based on the individual's specific needs. This precaution helps ensure the individual safely and effectively manages their asthma symptoms.
Regional Insights
North America dominated the market in 2023 due to various factors such as the availability of technologically advanced products, rising incidence of asthma, and increasing healthcare spending in the region. The region has witnessed a surge in the prevalence of asthma among the elderly population, particularly in the United States. This has contributed significantly to the North American market growth. The presence of major market players and manufacturers in the U.S. and the approval of critical products have further propelled the market growth. For instance, Cipla Limited received final approval for its Abbreviated New Drug Application (ANDA) for Albuterol Sulfate Inhalation Aerosol 90mcg (base)/actuation with a spacer from the United States Food and Drug Administration (US FDA) in April 2020. These continuous product approvals in the region are expected to strengthen North America's position in the asthma spacers market.
North America is witnessing an upward trend in the asthma spacers market, with new launches and advancements. In April 2023, GlaxoSmithKline Plc announced the launch of Breo AeroChamber Plus, a new addition to their portfolio of asthma spacer devices. The Breo AeroChamber Plus is explicitly designed to enhance the delivery of Breo Ellipta, a dry powder inhaler. This new product launch is expected to contribute significantly to the market growth in North America. Moreover, North America's dominance in the asthma spacers market can be attributed to various factors such as the presence of key market players, rising incidence of asthma, and increasing healthcare spending in the region. With continuous product launches and advancements, North America is expected to maintain its leading position in the asthma spacers market.
Competitive Insights
The global asthma spacer market is witnessing the emergence of major players such as Trudell Medical International, PARI Respiratory Equipment, Inc., Koninklijke Philips N.V, GlaxoSmithKline Plc., Lupin, Medical Developments International, Visiomed Group Ltd., Cipla Inc., Clement Clarke International Ltd., Rossmax International Ltd., Luckys Pharma, Medicare Equipments (India) Pvt. Ltd., Asia Connection Co., Ltd., and Laboratoire Protecsom SAS.














