- Home
- »
- Medical Devices
- »
-
Balloon Inflation Devices Market Size & Share Report, 2030GVR Report cover
![Balloon Inflation Devices Market Size, Share & Trends Report]()
Balloon Inflation Devices Market (2023 - 2030) Size, Share & Trends Analysis Report By Display Type (Analog, Digital), By End-use (Hospitals & Clinics, ASCs), By Application, By Region, And Segment Forecasts
- Report ID: GVR-4-68040-102-9
- Number of Report Pages: 100
- Format: PDF
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Interactive Charts
- Methodology
- Download FREE Sample
-
Download Sample Report
Report Overview
The global balloon inflation devices market size was estimated at USD 559.3 million in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 5.8% from 2023 to 2030. Balloon inflation devices allow the opening of narrowed or blocked blood vessels in the arteries to improve blood flow. The growing adoption of minimally invasive procedures, increasing prevalence of Cardiovascular Diseases (CVDs) across economies, and adoption of advanced technologies in the healthcare system are the key factors for market growth. In addition, the growing geriatric population is likely to develop coronary artery diseases, which propels the demand for balloon inflation devices as they are key in performing interventional cardiology operations. According to the National Institute of Health, an estimated 18.2 million Americans suffer from coronary artery disease.
The COVID-19 pandemic had a negative impact on the market. The supply chain was highly disrupted due to the pandemic. The COVID-19 epidemic impacted the overall logistics and shipping of balloon inflation device products across the globe, which resulted in high prices or low-product availability. Furthermore, non-urgent or elective procedures, including those involving balloon inflation devices, were delayed or postponed. Hospitals and healthcare facilities prioritized COVID-19 patients, leading to a decrease in the number of procedures performed. According to American Heart Association, Inc., a 49% fall was noticed in percutaneous coronary intervention (PCI) in the UK after March 2020. The increasing prevalence of CVDs across regions is anticipated to propel the market.
CVDs, such as coronary artery disease, peripheral artery disease, and valvular heart disease, require interventional procedures, such as angioplasty and stent placement, for treatment, aiding the use of balloon inflation devices. According to John Hopkins Medicine, an estimated 850,000 angioplasties are performed annually in the U.S. While according to American Heart Association Inc., an estimated 600,000 coronary stents are placed annually in the U.S. for percutaneous coronary interventions. In addition, the growing cases of cardiovascular diseases will provide lucrative opportunities for the market players. A positive reimbursement scenario adopted under Medicare positively impacts balloon inflation devices. For instance, in February 2020, Medicare announced to cover several types of PCI interventions as an outpatient service in the U.S.
The following regulatory change would allow the patients to undergo procedures, such as angioplasty, without hospitalization. In addition, the Centers for Medicare & Medicaid Services (CMS) estimated that shifting 5% of coronary interventions to ambulatory surgical centers (ASCs) from hospitals will result in savings of USD 20 million for CMS in 2020. The growing focus on Minimally Invasive Procedures (MIS) is anticipated to propel the market for balloon inflation devices. For instance, inflation devices are employed to treat urethral strictures, as these devices expand the narrowed urethra to restore urine flow. Similarly, the growing adoption of balloon inflation devices in radiology, urology, gynecology, and gastroenterology will propel market growth.
Display Type Insights
On the basis of display types, the global market has been further classified into analog and digital displays. The analog display segment dominated the market in 2022 and accounted for the maximum share of 62.07% of the overall revenue. The segment is estimated to grow further at the fastest CAGR of 6.1% retaining its leading industry position throughout the forecast period. The dominant share can be attributed to the high availability of these devices, established medical literature, and high preference amongst healthcare providers.
In addition, these devices are compatible with a wide range of balloon catheters, offering versatility in balloon size and type. They can also accommodate different clinical scenarios and procedural requirements. Moreover, key product manufacturers are launching analog-based inflation devices, which are compatible with different forms of balloon catheters. For instance, in November 2022, Merit Medical Systems, Inc. launched the basixALPHA inflation device in the U.S., which offers one-handed operation with faster inflation and minimal exertion properties, allowing streamlining angioplasty procedures.
Application Insights
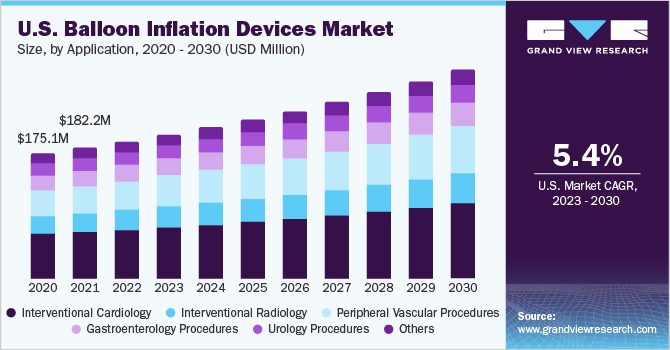
On the basis of applications, the global industry is classified into interventional cardiology, interventional radiology, peripheral vascular procedures, gastroenterology procedures, urology procedures, and others. The interventional cardiology segment dominated the market and accounted for a revenue share of 34.43% in 2022. Balloon inflation devices are extensively used in angioplasty procedures and stent placement. After the balloon angioplasty to open the narrowed artery, a stent is often deployed at the site to help keep the artery open and prevent re-narrowing.
According to the Eurostat article, an estimated 1 million transluminal coronary angioplasty procedures were performed in the EU in 2020. The peripheral vascular procedures are anticipated to witness the highest CAGR of 6.8% during the forecast period, owing to a growing number of atherectomy and thrombectomy procedures along with peripheral angioplasty procedures. According to the National Institute of Health, refractory stroke thrombectomy affects 8%-9% of the North American population.
End-use Insights
On the basis of end-uses, the global market is classified into hospitals, clinics, and ASCs. The hospital and clinics segment dominated the market and accounted for a revenue share of 69.06% in 2022. The segment is estimated to grow further at the fastest CAGR of 6.1% maintaining its dominant position throughout the forecast period. Hospitals and clinics are equipped with advanced infrastructure and facilities to perform a wide range of medical procedures, including interventional cardiology and peripheral vascular interventions.
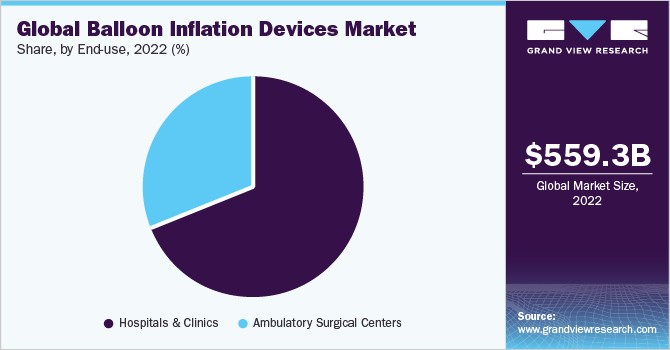
Moreover, hospitals and clinics promote collaboration among different medical specialties propelling the market growth. The ASCs end-use segment is also anticipated to grow at a significant rate during the forecast period. It is owing to the changing regulatory guidelines, reimbursement scenario, and growing government focus on improving patient care in developed countries, which allows for efficient care and low-cost treatment options in the ASC. Furthermore, an estimated USD 5.3 billion was spent by CMS for ASCs in 2022.
Regional Insights
North America dominated the global market and accounted for a revenue share of 37.41% in 2022. North America has a well-developed, advanced healthcare infrastructure, including hospitals, clinics, and medical facilities. Furthermore, the region has a high prevalence of CVDs, which drives the demand for balloon inflation devices. In addition, the region has a robust healthcare workforce, including interventional cardiologists, interventional radiologists, and vascular surgeons.
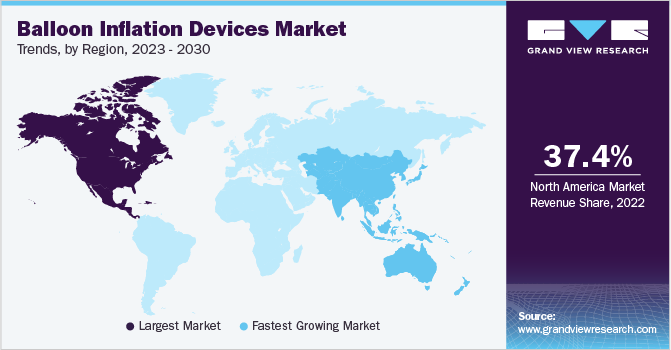
However, Asia Pacific is expected to witness the fastest CAGR of 7.2% over the forecast period. The growth is due to increasing healthcare spending, advancement in medical technology, and a growing number of CVDs in the geriatric population. Moreover, countries, such as China and India, are the leading medical device manufacturers which cater to the growing local demand. According to an article by the Times of India in November 2019, an estimated 450,000 angioplasties are performed each year in India.
Key Companies & Market Share Insights
Market players are introducing advanced products at affordable prices to increase their market share. Key players are implementing strategic initiatives, such as mergers, acquisitions, and collaborations, to maximize their market dominance. For instance, in June 2023, SYNDEO Medical launched the SATURN inflation device, which allows medical professionals to combine the benefits of different products in a singular platform. Some prominent players in the global balloon inflation devices market include:
-
Boston Scientific Corporation
-
Merit Medical Systems
-
CONMED Corporation.
-
Medtronic
-
Cook
-
Atrion Medical
-
BD
-
B. Braun Medical Inc.
-
US Endovascular, LLC
-
TZ Medical, Inc.
Balloon Inflation Devices Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 589.3 million
Revenue forecast in 2030
USD 877.15 million
Growth rate
CAGR of 5.8% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Display type, application, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; South Korea; Australia; Thailand; Brazil; Mexico, Argentina; South Africa; Saudi Arabia, UAE; Kuwait
Key companies profiled
TZ Medical, Inc.; US Endovascular, LLC; B. Braun Medical Inc.; BD; Atrion Medical; Medtronic; CONMED Corp.; Merit Medical Systems; Boston Scientific Corp.
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to Country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Balloon Inflation Devices Market Report Segmentation
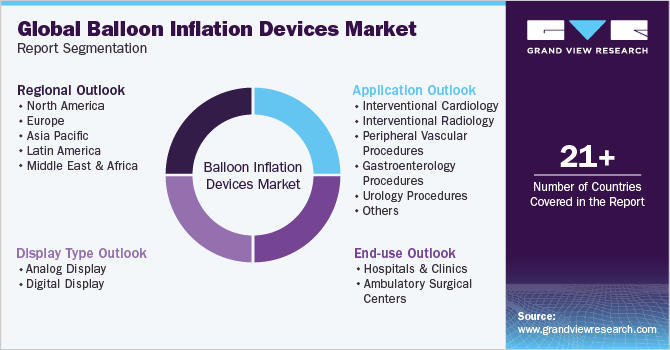
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the balloon inflation devices market report based on display type, application, end-use, and region:
-
Display Type (Revenue, USD Million, 2018 - 2030)
-
Analog Display
-
Digital Display
-
-
Application (Revenue, USD Million, 2018 - 2030)
-
Interventional Cardiology
-
Interventional Radiology
-
Peripheral Vascular Procedures
-
Gastroenterology Procedures
-
Urology Procedures
-
Others
-
-
End-use (Revenue, USD Million, 2018 - 2030)
-
Hospitals & Clinics
-
Ambulatory Surgical Centers
-
-
Regional Outlook (Revenue, USD Million 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Norway
-
Denmark
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global balloon inflation devices market size was estimated at USD 559.28 million in 2022 and is expected to reach USD 589.27 million in 2023.
b. The global balloon inflation devices market is expected to grow at a compound annual growth rate of 5.8% from 2023 to 2030 to reach USD 877.15 million by 2030.
b. North America dominated the balloon inflation devices market with a share of 37.41% in 2022. This is attributable to advanced healthcare facilities and the favorable medical reimbursement norms.
b. Some key players operating in the balloon inflation devices market include TZ Medical, Inc., US Endovascular, LLC., B. Braun Medical Inc., BD., Atrion Medical, Medtronic, CONMED Corporation., Merit Medical Systems., and Boston Scientific Corporation.
b. Key factors that are driving the balloon inflation devices market growth include increasing prevalence of cardiovascular diseases across regions and growing adoption of minimally invasive procedures.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










