Bone Growth Stimulators Market Trends
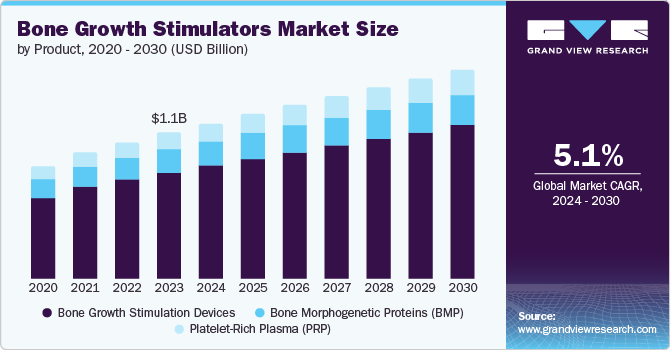
The global bone growth stimulators market size was valued at USD 1.07 billion in 2023 and is projected to grow at a CAGR of 5.1% from 2024 to 2030. The growth is fueled by the rising elderly population leading to more orthopedic disorders and injuries, higher occurrences of bone fractures and spinal injuries, growing popularity of minimally invasive treatments, and an increasing number of sports injuries. Furthermore, the rise in the penetration of hospitals and surgical centers and technological advancements are expected to drive the market growth.
The increasing prevalence of bone conditions such as fractures, osteoporosis, and osteoarthritis globally has led to a growing need for therapies that promote bone regeneration. Patients' desire for more effective treatments beyond traditional therapies drives the bone growth stimulator market. Osteoporosis affects around 200 million women globally, with approximately 1 in 10 women aged 60, 1 in 5 women aged 70, 2 in 5 women aged 80, and 2 in 3 women aged 90 being impacted.
The rise in sports-related injuries is expected to drive the market growth. In the U.S., around 30 million kids and adolescents join organized sports, resulting in over 3.5 million injuries annually. Athletes often use bone growth stimulators to speed up the recovery process, demonstrating their effectiveness in treating sports injuries.
Product Insights
Bone growth stimulation devices dominated the market in 2023. Factors such as the growing prevalence of bone and joint issues, increased need for minimally invasive and non-invasive procedures worldwide, and the expanding elderly population are driving the segment’s growth. The main advantage of a bone growth stimulator is its ability to accelerate the bone healing process, lower infection rates, reduced hospital stay, leading to quicker recovery and improved cost efficiency.
Platelet-rich plasma (PRP) is expected to register the fastest CAGR during the forecast period.
PRP provides a less invasive alternative to traditional bone grafting, resulting in less pain and shorter recovery periods for patients. Also, studies are confirming the effectiveness of PRP in enhancing bone healing for different conditions, creating trust and approval among surgeons.
Application Insights
Spinal fusion surgeries accounted for the largest market revenue share in 2023. This is due to various factors such as increased spinal disorders, the need for quicker healing and higher fusion rates for improved patient results, and decreased pain.
Orthopedic trauma surgeries are expected to register the fastest CAGR during the forecast period driven by various key factors. Primarily, there is a growing number of bone fractures and other orthopedic injuries worldwide due to increased involvement in sports, and a rise in traffic accidents. Additionally, bone growth stimulators can greatly enhance the speed of healing and lower the chances of complications following fractures, especially in complicated situations.
End-use Insights
Hospitals dominated the market in 2023 owing to increasing admission of patients suffering from traumatic injuries, road accidents, spinal injuries, and fractures, high patient turnaround and frequent readmissions for surgery and other treatments. Furthermore, increasing focus on the development of healthcare infrastructure in developing countries of Asia Pacific is expected to propel the growth of the segment. Rising disposable income and the availability of advanced medical devices for the treatment of different chronic disorders are expected to boost the usage of these products in hospitals.
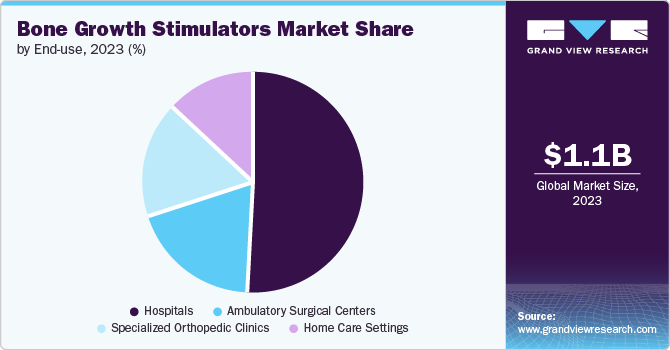
Specialized orthopedic clinics are anticipated to witness a substantial upsurge during the forecast period. These settings serve patients with sports injuries, fractures, and other bone-related conditions that may benefit from bone stimulation therapy. Moreover, the individualized care setting in specialized clinics enables close monitoring and adjustments to bone stimulation therapy for best outcomes.
Regional Insights
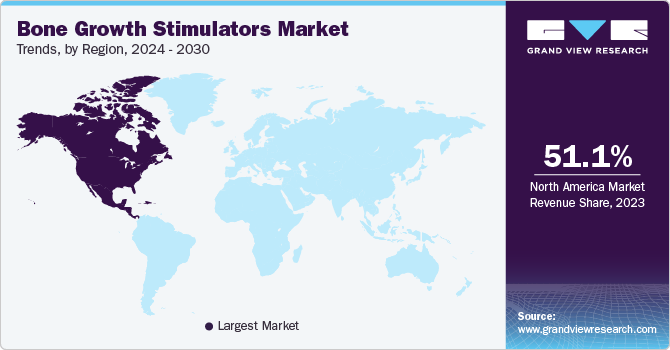
North America bone growth stimulators market dominated the market in 2023 due to frequent occurrences of orthopedic and spinal issues such as bone fractures and spinal injuries, improved therapeutic methods and enhanced treatment procedures.
U.S. Bone Growth Stimulators Market Trends
The U.S. bone growth stimulators market dominated the North America in 2023 due to advanced healthcare system in the U.S. The combination of increasing healthcare costs and penetration on minimally invasive techniques are factors propelling the market growth.
Europe Bone Growth Stimulators Market Trends
Europe bone growth stimulators market was identified as a lucrative region in 2023 due to growing geriatric population base. Aging population with bone disorders such as osteoporosis generates a substantial need for these devices and therapies. Furthermore, Europe has a strong healthcare system that prioritizes enhancing patient results.
The UK bone growth stimulators market is expected to grow rapidly in the coming years due to an increase in medical tourism and a rising adoption of minimally invasive procedures.
Asia Pacific Bone Growth Stimulators Market Trends
Asia Pacific bone growth stimulators market is anticipated to witness significant growth in the coming years. The bone growth stimulant market is significantly being driven by the developing nations like India, China, and Japan, among other countries.
The bone growth stimulators market in India is expected to grow rapidly in the coming years. Advancements in the nation's healthcare sector are fueling expansion. Additionally, government policies/schemes are expected to have a positive impact on market growth.
The China bone growth stimulators market held a substantial market share in 2023.
The rise in cervical and lumbar spine surgeries emphasizes the importance of advanced combined magnetic field (CMF) devices for bone growth stimulation therapy.
Key Bone Growth Stimulators Company Insights
Some of the key companies in the bone growth stimulators market include Orthofix Medical Inc., Medtronic, Zimmer Biomet and others. Organizations are focusing on increasing customer base to gain a competitive edge in the industry. Therefore, key players are taking several strategic initiatives, such as mergers and acquisitions, and partnerships with other major companies.
Key Bone Growth Stimulators Companies:
The following are the leading companies in the bone growth stimulators market. These companies collectively hold the largest market share and dictate industry trends.
- Orthofix Holdings, Inc.
- Medtronic
- Zimmer Biomet
- DePuy Synthes
- Arthrex, Inc.
- Bioventus
- DJO Global, Inc.
- Ossatec benelux
- BTT Health GmbH
- IGEA
- Isto Biologics
Recent Developments
-
In June 2024, Johnson & Johnson MedTech announced that DePuy Synthes received FDA clearance for VELYS Robotic-Assisted Solution in Unicompartmental Knee Arthroplasty (UKA) clinical application. The application is for medical & lateral procedures and is expected to help surgeons in guiding precise implant placement with no CT scan.
-
In April 2024, Xstim announced that it received premarket approval for Xstim spine fusion stimulator from the U.S. FDA. It is a non-invasive, wearable bone growth stimulation device which emits low-energy signal promoting bone healing after spinal fusion surgery of patients.
Bone Growth Stimulators Market Report Scope
|
Report Attribute
|
Details
|
|
Market size value in 2024
|
USD 1.14 billion
|
|
Revenue forecast in 2030
|
USD 1.53 billion
|
|
Growth rate
|
CAGR of 5.1% from 2024 to 2030
|
|
Base year for estimation
|
2023
|
|
Historical data
|
2018 - 2022
|
|
Forecast period
|
2024 - 2030
|
|
Quantitative units
|
Revenue in USD billion and CAGR from 2024 to 2030
|
|
Report coverage
|
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
|
|
Segments covered
|
Product, application, end-use, region
|
|
Regional scope
|
North America, Europe, Asia Pacific, Latin America, MEA
|
|
Country scope
|
U.S., Canada, Mexico, Germany, UK, France, Spain, Denmark, Sweden, Norway, China, Japan, India, South Korea, Australia, Thailand, Brazil, Argentina, KSA, UAE, South Africa
|
|
Key companies profiled
|
Orthofix Holdings; Inc. Medtronic; Zimmer Biomet; DePuy Synthes; Arthrex, Inc. Bioventus ;DJO Global, Inc.;Ossatec Benelux; BTT Health GmbH; IGEA; Isto Biologics.
|
|
Customization scope
|
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
|
|
Pricing and purchase options
|
Avail customized purchase options to meet your exact research needs. Explore purchase options
|
Global Bone Growth Stimulators Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global bone growth stimulators market report based on product, application, end-use, and region.
-
Product Outlook (Revenue, USD Billion, 2018 - 2030)
-
Bone growth stimulation devices
-
Bone Morphogenetic Proteins (BMP)
-
Platelet-Rich Plasma (PRP)
-
Application Outlook (Revenue, USD Billion, 2018 - 2030)
-
End-use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)















