- Home
- »
- Medical Devices
- »
-
Cardiac Rhythm Management Devices Market Report, 2033GVR Report cover
![Cardiac Rhythm Management Devices Market Size, Share & Trends Report]()
Cardiac Rhythm Management Devices Market (2025 - 2033) Size, Share & Trends Analysis Report By Product (Pulse Generators, Leads), By End-use (Hospitals, Ambulatory Surgery Centers), By Region, And Segment Forecasts
- Report ID: GVR-2-68038-315-7
- Number of Report Pages: 250
- Format: PDF
- Historical Range: 2021 - 2024
- Forecast Period: 2025 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Cardiac Rhythm Management Devices Market Summary
The global cardiac rhythm management devices market size was estimated at USD 20.77 billion in 2024 and is projected to reach USD 36.19 billion by 2033, growing at a CAGR of 6.35% from 2025 to 2033. Growing prevalence of various cardiovascular diseases, such as arrhythmia and atrial fibrillation, among others.
Key Market Trends & Insights
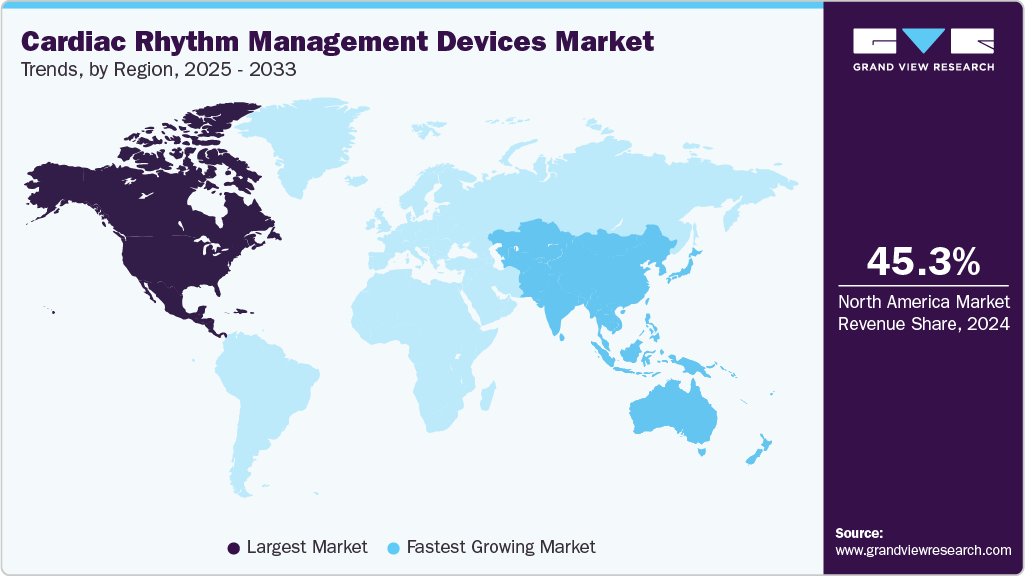
- North America cardiac rhythm management devices market dominated global market in 2024 with a revenue share of 45.34%.
- The U.S. cardiac rhythm management devices market accounted for the largest share in North America in 2024.
- Based on product, the pulse generators segment held the largest share of 79.46% in 2024.
- Based on end-use, the hospitals segment accounted for the largest revenue share of 53.49% in 2024.
Market Size & Forecast
- 2024 Market Size: USD 20.77 Billion
- 2033 Projected Market Size: USD 36.19 Billion
- CAGR (2025-2033): 6.35%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
According to the Centers for Disease Control and Prevention (CDC), around 12.1 million people in the U.S. are expected to suffer from atrial fibrillation (AFib) by 2030. Furthermore, various technological advancements related to the market such as integration of AI, remote monitoring, and wearable technology, are anticipated to fuel the market growth.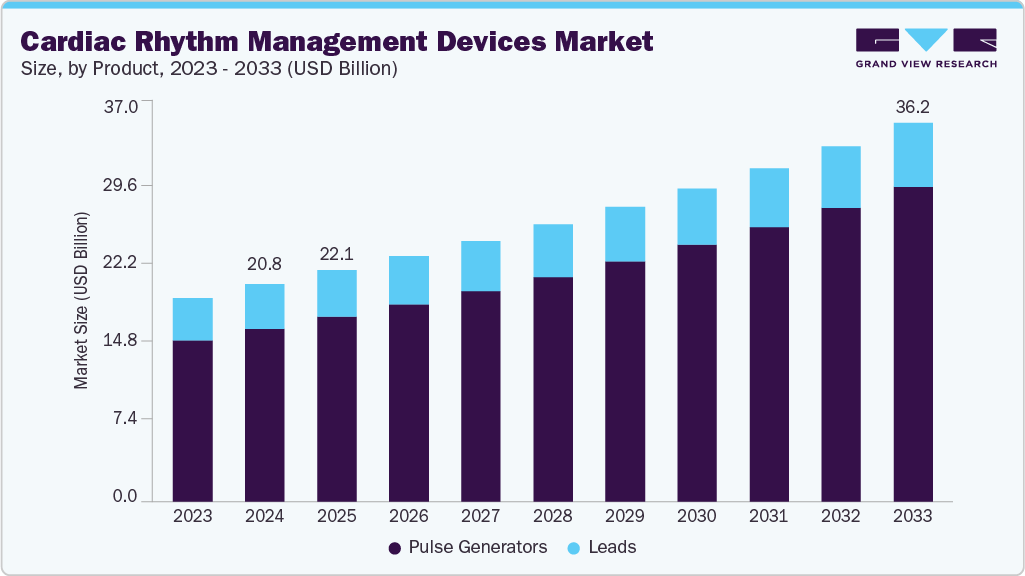
The widespread impact of cardiovascular disease (CVD) is a key aspect boosting the growth and innovation within the cardiac monitoring and cardiac rhythm management devices market. According to an Oxford Academic article published in January 2025, the global age-standardized prevalence of cardiovascular disease reaches about 7,179 cases per 100,000 people, underscoring the significant and ongoing impact of heart conditions globally. Such widespread prevalence means that millions are living with diseases that require careful monitoring and timely intervention to prevent complications. This fuels demand for cardiac monitoring and cardiac rhythm management devices, critical for identifying abnormal heart rhythms, managing chronic heart conditions, and reducing the risk of serious cardiac events
Key Facts on Cardiovascular Disease and Related Events in the U.S. (2024)
Statistic
Value
Total CVD deaths per day
2,552
Deaths from heart disease per day (including heart attacks)
1,905
Average time between heart attacks
Every 40 seconds
New heart attacks each year
Approximately 605,000
Recurrent heart attacks each year
Approximately 200,000
Silent heart attacks
Estimated 170,000
Average age at first heart attack (males)
65.6 years
Average age at first heart attack (females)
72.0 years
Source: American Heart Association, Inc. in January 2024 & GVR
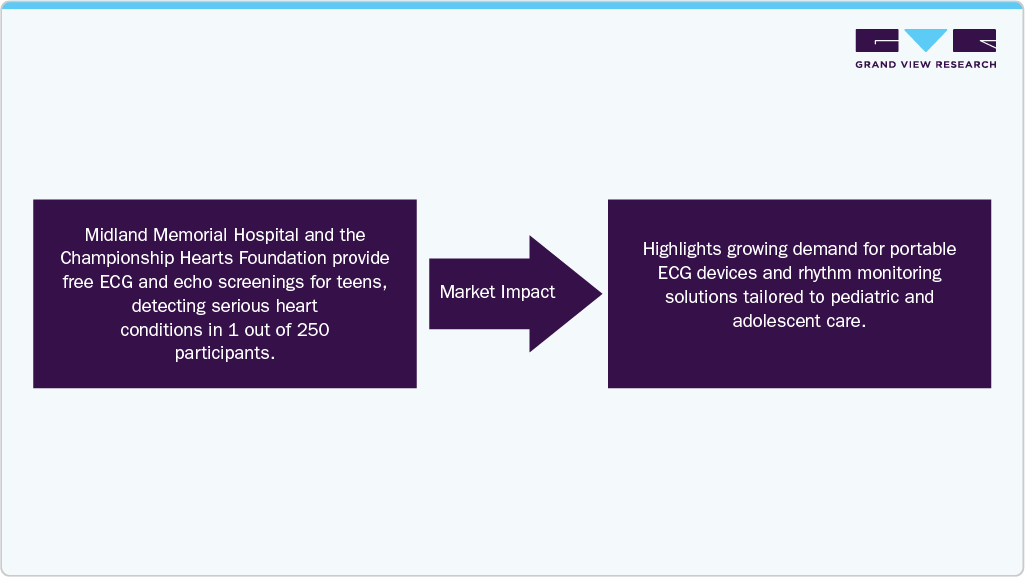
Increasing awareness campaigns and government-initiated screening programs for early detection of CVDs contribute to market expansion by ensuring more patients receive timely diagnosis and treatment. According to an article published by Hearst Newspapers II, LLC in June 2025, Midland Memorial Hospital, working alongside the Championship Hearts Foundation, conducts free ECG and limited echocardiogram screenings for teenagers between 14 and 18 years old. In previous events, these screenings identified severe, potentially life-threatening heart conditions, such as hypertrophic cardiomyopathy, in approximately 1 out of every 250 participants. Initiatives like this highlight the critical role of early detection in preventing sudden cardiac events among young people.
The technologically advanced products offered by the key players drive the market's growth. For instance, in September 2025, Biotronik launched Solia CSP S, a pacing lead combining a fixed screw design with a stylet-driven implantation approach. It is designed specifically for conduction system pacing (CSP) to simplify implant handling without compromising outcomes.
“The launch of Solia CSP S represents a step forward in making CSP procedures more accessible and efficient. It is part of our ongoing commitment to physiologic pacing and providing physicians with tools that streamline implantation for better patient outcomes.” -President CRM/EP at BIOTRONIK.
Similarly, in November 2024, Abbott launched the AVEIR VR leadless pacemaker in India to help patients with unusually slow heart rates. The device has received approval from India’s Central Drugs Standard Control Organization (CDSCO). It has also been cleared by the U.S. Food and Drug Administration (FDA), representing a notable step forward in cardiac care by offering innovative features for patients and doctors. For individuals whose heartbeats are too slow, pacemakers deliver electrical impulses that help maintain a proper heartbeat. Unlike traditional pacemakers, which require a surgical pocket in the chest and wires called leads to connect to the heart, leadless pacemakers like AVEIR VR are implanted directly into the heart, eliminating the need for leads or a chest pocket.
Technological trends in the cardiac rhythm management devices market
Segment
Key Innovations & Features
Clinical / Patient Benefits
Notable Examples / Companies
CRT (CRT-D, CRT-P)
Bluetooth-enabled remote monitoring, adaptive pacing algorithms (e.g., AdaptivCRT), leadless CRT, multipoint pacing
Early detection of device issues, improved synchronization, fewer hospital visits, enhanced therapy outcomes
Medtronic, Boston Scientific
Implantable Defibrillators (ICDs)
Complete analysis and detailed commentary will be included in Final Deliverable
Pacing Devices (Pacemakers)
Leads (Pacing & Defibrillator Leads)
Source: Company websites, Secondary Research, Grand View Research Analysis
Emerging Technologies
Breakthroughs in wireless left ventricular pacing and conduction system pacing (CSP) are redefining CRM therapies.
Technologies such as EBR Systems’ WiSE CRT System use ultrasound-based energy transfer for leadless LV pacing-offering better CRT response and fewer complications.
Meanwhile, CSP and Left Bundle Branch Area Pacing (LBBAP) enable natural conduction activation, improving synchronization and outcomes in heart failure patients.WiSE CRT System - EBR Systems, Inc.
-
Technology: First FDA-approved (April 2025) leadless LV endocardial pacing system using ultrasound-based stimulation.
-
Clinical Impact: Achieved 80.9% freedom from major complications and 16.4% LV volume reduction in JAMA Cardiology trial (2024).
-
Regulatory Milestone: Received CMS Transitional Pass-Through reimbursement (Oct 2025), expanding hospital adoption.
-
Significance: Offers a disruptive, lead-free CRT alternative for heart failure patients with complex anatomies.
Conduction System Pacing (CSP)
-
Overview: Engages native conduction pathways for physiological ventricular activation.
-
Advantage: Demonstrates superior synchrony and functional improvement over traditional biventricular pacing (BVP).
-
Adoption: Rapidly gaining acceptance for bradycardia and heart failure indications as regulatory approvals expand.
Product Pipeline Analysis
The pipeline for Cardiac Rhythm Management (CRM) and monitoring devices is vast, with around 148 devices in development. Out of these, 74 are actively being developed, including 42 early-stage and 32 late-stage devices. The majority of these are expected to gain regulatory approval within the next decade.
Key Innovations in CRM Pipeline
-
Leadless & Physiological Implants:
-
Significant push towards dual-chamber leadless pacemakers (e.g., Abbott’s AVEIR DR, FDA-approved in July 2023) and extravascular ICDs (e.g., Medtronic’s Aurora EV ICD MRI SureScan).
-
Temporary & Dissolvable Pacemakers:
-
Northwestern and University of Chicago are working on biodegradable pacemakers designed for short-term use, dissolving naturally post-treatment.
-
Remote Monitoring & Wearables:
-
Advancements in insertable cardiac monitors (ICM) such as Boston Scientific’s LUX-Dx II+ and Abbott’s Assert IQ, alongside consumer wearables like CardiacSense and AliveCor, that provide continuous ECG, SpO₂, and AF detection.
Pipeline Product Table
Device / Category
Company / Developer
Stage
Notes
Leadless dual chamber pacemaker (AVEIR DR)
Abbott
Late / Commercial
FDA-approved July 2023
Complete analysis and detailed commentary will be included in Final Deliverable.
Competitive Factors and Strategies in the Cardiac Rhythm Management Devices Market
Product Improvements & Innovations
Continuous innovation in physiological pacing, MRI-compatible devices, miniaturization, and wireless connectivity is reshaping the cardiac rhythm management (CRM) landscape.
Leading companies are advancing Bluetooth-enabled ICDs, intelligent remote monitoring, and conduction system pacing technologies to deliver safer, patient-centric cardiac therapies. For instance, MicroPort Scientific launched next-generation lead systems, LBBAP kits, and MRI-safe Bluetooth ICDs, enhancing clinical precision and patient outcomes.Key Focus Areas:
-
Lead Technologies: Refined pacing leads (e.g., Vega) improve durability and conduction stability.
-
Physiological Pacing (LBBAP): Advanced delivery systems (Flexigo Kit, Falcon6, LINEA LBBAP) enable natural cardiac synchrony and reduce complications.
-
ICDs: Bluetooth Low Energy MRI ICDs (Tilen, Eylen) support remote monitoring and imaging safety.
-
Connectivity: Devices like Smarview Connect and Alizea Bluetooth enable continuous wireless data flow for proactive care.
-
Regulatory Focus: MRI compatibility and cybersecurity ensure compliance and global market readiness.
Market Developments and Key Clinical Trials
The CRM market is evolving rapidly with dual-chamber leadless pacemakers, CSP advancements, and AI-driven remote monitoring reshaping device adoption.
Clinical data consistently favor LBBAP for enhanced synchrony and reduced heart failure hospitalizations compared to conventional BVP.Recent Late-Breaking Trials (Heart Rhythm 2025)
Trial
Key Insight
I-CLAS Global Registry
LBBAP reduced death/HF hospitalization vs BVP (22.2% vs 30.8%); improved CRT outcomes.
LEADR LBBAP Study
Demonstrated 88% efficacy; 100% defibrillation success; validated OmniaSecure lead versatility.
Source: Heart Rhythm Society, Grand View Research Analysis
Key Ongoing Clinical Trials
Focus Areas: CRT optimization, leadless pacing, conduction system activation, and AI-based remote monitoring.
Representative trials include:-
NCT05434962 - The Left Bundle CRT Trial: Evaluates physiological pacing outcomes in LBBB & HF.
-
NCT05659680 - WiSE-CRT: Tests ultrasound-based leadless pacing safety and efficacy.
- NCT04774523 - BIOTRONIK AutoAdapt: Studies adaptive CRT algorithm for continuous resynchronization.
Market Concentration & Characteristics
The cardiac rhythm management devices industry exhibits a high degree of innovation, driven by advancements in leadless pacemakers, subcutaneous ICDs, and AI-enabled remote monitoring systems. These technologies improve patient safety, reduce procedural complications, and extend device longevity. Adaptive pacing and predictive arrhythmia detection enable more personalized therapy for diverse cardiac conditions. Integration with digital health platforms allows real-time monitoring and remote care. Continuous innovation ensures devices remain patient-centric while meeting evolving clinical demands. Manufacturers are also investing in miniaturization and energy-efficient designs to enhance patient comfort and device performance.
Companies in the cardiac rhythm management devices industry actively pursue mergers, acquisitions, and strategic partnerships at medium level to strengthen technological capabilities and market reach. Collaborations often focus on integrating AI analytics, remote monitoring solutions, and next-generation device designs. M&A activity accelerates product development, diversifies portfolios, and provides a competitive edge in high-growth regions. Such partnerships facilitate access to regulatory expertise and established distribution networks. Overall, consolidation and strategic alliances are shaping the competitive landscape and driving faster adoption of advanced therapies.
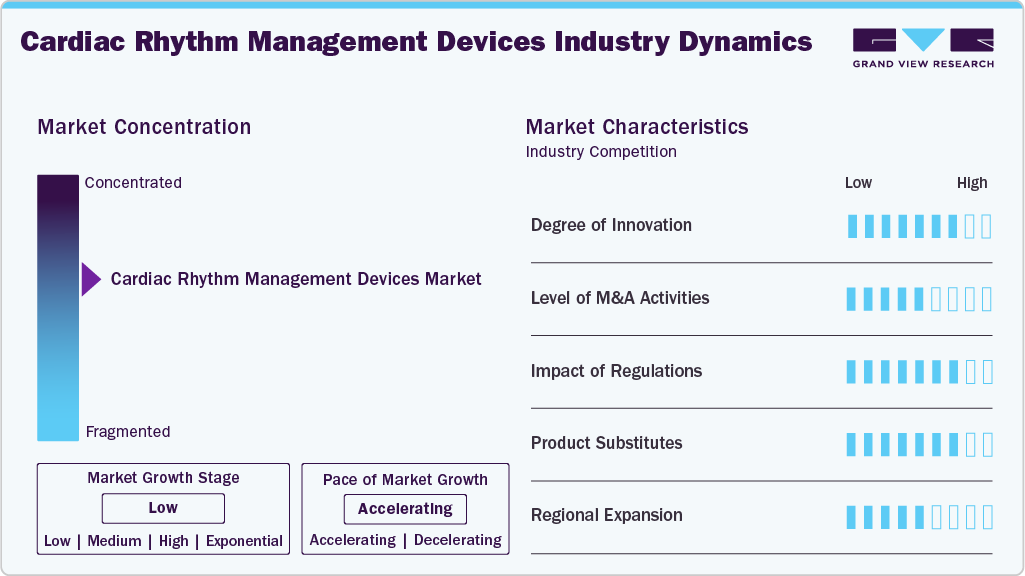
Cardiac rhythm management devices industry is governed by strict regulatory frameworks, including FDA approval in the U.S., CE marking in Europe, and local medical device regulations worldwide. Compliance ensures device safety, clinical efficacy, and cybersecurity, while influencing development costs and market entry timelines. Regulatory agencies are increasingly accommodating AI-enabled diagnostics and remote monitoring technologies. In May 2023, MicroPort Scientific Corporation secured FDA approval for its Alizea and Celea pacemaker systems, currently the most durable pacemakers available in their size class. These devices feature Bluetooth connectivity and AutoMRI functionality, enabling automated MRI mode switching.
Manufacturers are expanding product portfolios in the cardiac rhythm management devices industry at high rate through enhancements such as adaptive pacing, wireless monitoring, miniaturized designs, and improved battery life. These upgrades aim to address a broader range of cardiac conditions and improve patient comfort. Integration with telehealth platforms supports remote follow-up, therapy optimization, and real-time arrhythmia management. In October 2023, MicroPort CRM, has announced the introduction of the ULYS Implantable Cardioverter Defibrillator (ICDs) and the INVICTA defibrillation lead in the Japanese market, aiming to strengthen their product portfolio across the globe.
Key players in the cardiac rhythm management devices industry are expanding into Asia-Pacific, MENA, and Latin America at medium strength to capture high-growth opportunities and address rising cardiac disease prevalence. Expansion strategies include establishing distribution networks, clinician training programs, and remote patient monitoring services. These efforts improve therapy access, adherence, and outcomes in underserved regions. In December 2023, Medtronic officially introduced its Penditure Left Atrial Appendage (LAA) Exclusion System in the U.S.
Product Insights
By product, the pulse generators segment accounted for the largest revenue share of 79.46% in 2024. The segment growth is propelled by the rising incidence of cardiovascular diseases, especially arrhythmia and heart failure and technologically advanced product launch. Pulse generators are designed to regulate heart rhythm in patients with arrhythmias, bradycardia, or heart failure. Remote programming capabilities and device telemetry allow clinicians to make parameter changes and monitor device performance without requiring in-clinic visits, enabling more personalized and responsive care pathways for chronically ill patients. For instance, in March 2025, MicroPort Scientific Corporation gained CE Mark approval for its SmartView Connect App Mobile, allowing Android-based remote monitoring of Bluetooth-enabled pacemakers, ICDs, and CRT-Ds. The app securely transmits encrypted data to the SmartView network, replacing bedside monitors and supporting real-time, clinic-connected cardiac care across Europe.
The leads segment is expected to grow at significant CAGR over the forecast period. Leads are essential components of pacemakers and implantable cardioverter-defibrillators (ICDs). They transmit electrical impulses between the device and cardiac tissue. Pacemaker leads maintain heart rhythm by delivering pacing stimuli, while defibrillators provide high-voltage shocks to terminate life-threatening arrhythmias. Furthermore, regulatory approvals and advancements in healthcare infrastructure support segment expansion by increasing access to advanced pacing technologies worldwide. For instance, in September 2024, Boston Scientific Corporation received FDA approval for expanded indication of its INGEVITY+ Pacing Leads to include conduction system pacing (CSP) and sensing of the left bundle branch area (LBBA) when connected to single or dual-chamber pacemakers. As growing evidence emphasizes its safety, practicality, and improved clinical outcomes compared to traditional pacing methods, its broader usage is anticipated, underscoring its significance in modern physiological pacing strategies.
End-use Insights
By end use, the hospitals segment accounted for the largest revenue share of 53.49% in 2024. Hospitals remain the primary environment for the utilization and integration of cardiac rhythm management devices due to their ability to manage high-acuity patients effectively and provide device-based interventions. Necessary devices in this field include pacemakers, ICDs, and CRT devices. Continuous cardiac monitoring is essential for patients in emergency, intensive care, and perioperative settings. It enables the early detection of arrhythmias, ischemic events, or heart failure decompensation, allowing for timely interventions.
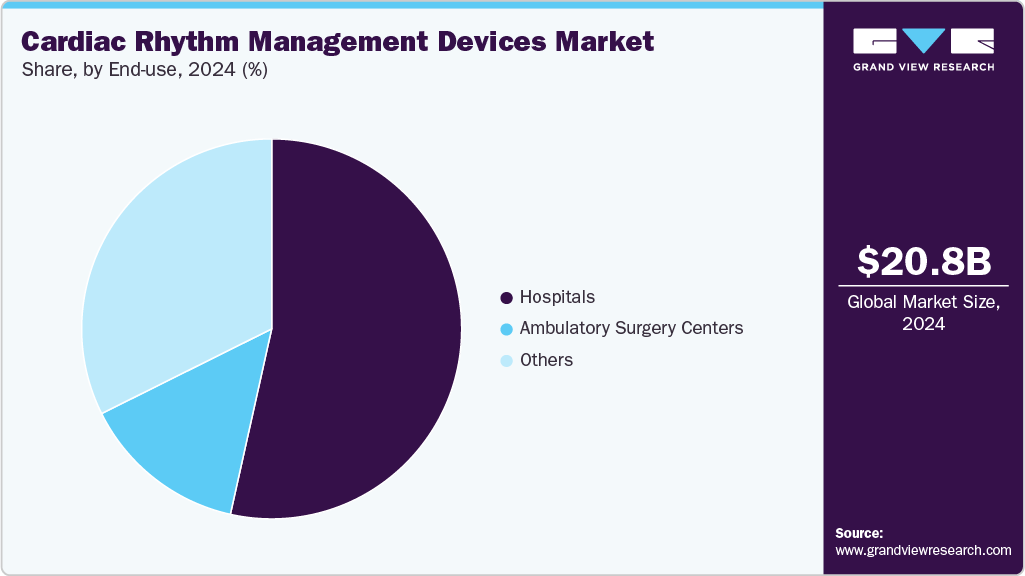
The ambulatory surgery centers segment is expected to grow at fastest CAGR over the forecast period. ASCs play a significant role in the procedural aspects of cardiac rhythm management, especially for patients undergoing low-risk pacemaker or loop recorder implantation. This transition towards ASCs is part of a broader initiative to alleviate the burden on inpatient services and reduce procedural costs, all while ensuring favorable clinical outcomes. Typically, these centers focus on stable patients and utilize minimally invasive methods, aided by portable ECG and device programming technologies, to efficiently conduct device implants and diagnostics. For instance, in February 2024, the American College of Cardiology unveiled its CV ASC Registry Suite to track the growing number of cardiac procedures performed in these outpatient facilities. The registry monitors interventions, including diagnostic catheterizations and the implantation of pacemakers and defibrillators.
Regional Insights
North America cardiac rhythm management devices market dominated the global market with a share of 45.34% in 2024. This growth is attributed to the robust healthcare infrastructure already in place, the growing elderly population, the swift acceptance of advanced technology-driven products such as those offering extended battery life, biocompatibility, miniaturization, and leadless designs, and the escalating number of regulatory approvals. According to the American Heart Association article published in March 2025, Atrial fibrillation (AFib), the most prevalent form of irregular heart rhythm, affected about five million individuals across the U.S. in 2024.
U.S. Cardiac Rhythm Management Devices Market Trends
The U.S. cardiac rhythm management devices market accounted for the largest market share in North America in 2024. The U.S. has a well-established healthcare infrastructure with a high prevalence of cardiovascular diseases, contributing to a robust demand for cardiac rhythm management devices. In December 2024, HeartBeam, Inc., a medical technology firm dedicated to advancing cardiac care through innovative diagnostic tools, recently announced that it has secured 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its HeartBeam system, designed for comprehensive arrhythmia detection.
Europe Cardiac Rhythm Management Devices Market Trends
The launch of new products in Europe cardiac rhythm management devices market is expected to drive the market due to the increasing prevalence of cardiovascular diseases and the rise in the geriatric population. These new devices offer improved accuracy & efficiency in monitoring and treating cardiac rhythm disorders. Technological advancements and increasing investments in R&D by key players in the industry are expected to drive market growth further. In January 2024, MicroPort Scientific Corporation announced the CE mark approvals for TALENTIA and ENERGYA. These devices monitor the heart rhythm and deliver electrical shocks to regulate abnormal heartbeats if necessary.
The cardiac rhythm management devices market in the UK is anticipated to grow at a significant rate over the forecast period. This can be attributed to the increasing prevalence of cardiac disorders, such as atrial fibrillation and congenital heart defects, which require the use of EP devices for diagnosis & treatment. According to the British Heart Foundation article published in January 2025, heart and circulatory diseases affect over 7.6 million people in the UK, with more than four million men and around 3.6 million women living with these conditions.
The cardiac rhythm management devices market in Germany has been driven by technological advancements, which have led to the development of more effective & safe devices. Technological advancements have enabled the development of more advanced devices that offer better monitoring, diagnostic capabilities, and treatment options for patients with cardiac conditions.According to the Georg Thieme Verlag KG article published in August 2024, Germany recorded 168,841 cardiac-related procedures, with over 100,000 classified as traditional heart surgeries in 2023.
Asia Pacific Cardiac Rhythm Management Devices Market Trends
The Asia Pacific cardiac rhythm management devices market is expected to grow fastest during the forecast period.Growth is driven by increasing cardiovascular disease prevalence, rising patient awareness, and expanding access to advanced cardiac care. Rapid adoption of leadless pacemakers and subcutaneous ICDs is fueling demand. Supportive government initiatives, improving healthcare infrastructure, and investments by global players are accelerating market expansion in China, India, Japan, and Australia. In February 2024, MicroPort CRM, a prominent player in cardiac rhythm management, launched its GALI SonR CRT-D device and NAVIGO 4LV left ventricular pacing leads in Japan, marking a significant step forward in advanced heart failure treatment.
China cardiac rhythm management devices market accounted for the largest market share in the Asia Pacific region in 2024. China has experienced rapid economic growth, resulting in an expanding healthcare sector with increased investments in advanced medical technologies, including cardiac rhythm management devices. The country's large aging population contributes to a higher incidence of cardiovascular diseases, driving the demand for such devices.According to the NCBI article published in December 2023, China is witnessing a rising prevalence of atrial fibrillation, with 4.87 million individuals currently affected by this common cardiac arrhythmia
Australia cardiac rhythm management devices market is expected to grow at a significant CAGR over the forecast period.Growth is driven by rising prevalence of cardiovascular diseases, increasing adoption of advanced devices such as leadless pacemakers and subcutaneous ICDs, and expanding cardiac care infrastructure. According to the Heart Foundation article published in March 2024, in Australia, one in six individuals self-report as living with cardiovascular disease (CVD), amounting to over 4.5 million people. This significant figure represents nearly 18% of the total Australian population.
Latin America Cardiac Rhythm Management Devices Market Trends
The cardiac rhythm management market in Latin America is expected to witness significant growth over the forecast period, driven byincreasing prevalence of cardiovascular diseases, rising patient awareness, and expanding access to advanced cardiac care facilities. Adoption of leadless pacemakers, subcutaneous ICDs, and remote monitoring technologies is accelerating demand. According to an NCBI article published in December 2024, over 22,000 cardiac rhythm management devices were implanted in Argentina in 2022, highlighting the growing reliance on advanced cardiac technologies.
Middle East & Africa Cardiac Rhythm Management Devices Market Trends
The Cardiac Rhythm Management Devices market in the MEA is growing significantly due torising prevalence of cardiovascular diseases, increasing patient awareness, and expansion of cardiac screening programs. Adoption of advanced devices such as leadless pacemakers and subcutaneous ICDs is accelerating demand. Investments in healthcare infrastructure, clinician training, and government initiatives supporting early detection and treatment are further driving market growth across countries such as Saudi Arabia, UAE, and South Africa. In May 2024, Bayer and Huma Therapeutics Limited launched an advanced heart health screening tool in Saudi Arabia. This is the tool's first international release after the Bayer Aspirin Heart Health Risk Assessment debuted in the U.S. in 2023.
Key Cardiac Rhythm Management Devices Company Insights
Leading players in the cardiac rhythm management market such as Boston Scientific Corporation, Medtronic and Abbott have strategically employed innovative approaches, including mergers and acquisitions, market penetration initiatives, partnerships, and distribution agreements. These strategies aim to enhance their revenue streams by leveraging collaborative efforts, expanding market reach, and fostering synergies within the dynamic landscape of cardiac rhythm management. For instance, in June 2023, Zahrawi Group is excited to unveil its collaboration with AppSens, a Norwegian firm, to distribute the cutting-edge cardiac monitoring solution, ECG247, throughout the Kingdom of Saudi Arabia.
Emerging market entrants such as Progetti Srl and, LivaNova Plc are directing their efforts toward broadening their market presence, creating inventive technologies, and establishing strategic partnerships as part of their strategy to contend with established industry leaders.
Key Cardiac Rhythm Management Devices Companies:
The following are the leading companies in the cardiac rhythm management devices market. These companies collectively hold the largest Market share and dictate industry trends.
- Abbott
- Medtronic
- Boston Scientific Corporation
- Biotronik
- Schiller
- Koninklijke Philips N.V.
- MicroPort Scientific Corporation
- Lepu Medical Technology (Beijing) Co., Ltd.
- Stryker
Recent Developments
-
In September 2025, BIOTRONIK launched Solia CSP S, a pacing lead combining a fixed screw design with a stylet-driven implantation approach. It is designed specifically for conduction system pacing (CSP) to simplify implant handling without compromising outcomes.
-
In September 2025, Boston Scientific received CE Mark approval to expand the indication of its INGEVITY+ Pacing Leads to include conduction system pacing (CSP) and sensing in the left bundle branch area (LBBA) when connected to single- or dual-chamber pacemakers.
-
In March 2025, MicroPort Scientific Corporation launched the localized TEN pacemaker family in China through its affiliate MicroPort CRM Shanghai. The TEN series includes six compact, long-life models across T, E, and N categories, offering AutoMRI compatibility and the SAM function for continuous sleep and arrhythmia monitoring.
-
In June 2024, Stryker launched the LIFEPAK 35 monitor/defibrillator to support advanced life support in both EMS and hospital settings. The device features an intuitive touchscreen, real-time data connectivity, and ergonomic design. It aims to reduce cognitive burden and improve response efficiency for frontline providers.
-
In January 2024, Medtronic joined forces with Cardiac Design Labs to introduce Padma Rhythms, an External Loop Recorder designed for extended heart rhythm monitoring in India. As the exclusive distributor, Medtronic aims to broaden remote diagnostic access. The device integrates AI-powered dual-channel ECG and enables real-time data transmission.
Cardiac Rhythm Management Devices Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 22.12 billion
Revenue forecast in 2033
USD 36.19 billion
Growth rate
CAGR of 6.35% from 2025 to 2033
Actual data
2021 - 2024
Forecast period
2025 - 2033
Quantitative units
Volume in thousand units, revenue in USD billion/million and CAGR from 2025 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand; South Korea; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Abbott; Medtronic; Boston Scientific Corporation; Biotronik; Schiller; Koninklijke Philips N.V.; MicroPort Scientific Corporation; Lepu Medical Technology (Beijing) Co., Ltd.; Stryker
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options Global Cardiac Rhythm Management Devices Market Report Segmentation
This report forecasts revenue growth at global, regional & country levels and provides an analysis of the industry trends in each of the sub-segments from 2021 to 2033. For this study, Grand View Research has segmented the global cardiac rhythm management devices market report based on product, end-use and region:
-
Product Outlook (Unit Volume in ‘000 Units; Average Selling Price [USD per Unit]; Revenue in USD Million, 2021 - 2033)
-
Pulse Generators
-
Cardiac Resynchronization Therapy (CRT)
-
CRT-Defibrillator
-
CRT-Pacemakers
-
-
Defibrillators
-
Implantable Cardioverter Defibrillators (ICD)
-
Transvenous Implantable Cardioverter Defibrillators (TIICDs)
-
Single Chamber
-
Dual Chamber
-
-
Subcutaneous Implantable Cardioverter Defibrillators (S-ICDs)
-
External Defibrillator.
-
-
Pacemakers
-
Conventional Pacemakers
-
Leadless Pacemakers
-
-
-
Leads
-
Pacing Leads
-
Standard RV/LV Leads
-
Conduction System Pacing (CSP) / LBBAP Leads
-
-
Defibrillator Leads
-
Traditional Leads
-
LV Leads
-
-
-
-
End-use Outlook (Unit Volume in ‘000 Units; Average Selling Price [USD per Unit]; Revenue in USD Million, 2021 - 2033)
-
Hospitals
-
Ambulatory Surgery Centers
-
Others
-
-
Regional Outlook (Unit Volume in ‘000 Units; Average Selling Price [USD per Unit]; Revenue in USD Million, 2021 - 2033)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global cardiac rhythm management devices market size was estimated at USD 14.41 billion in 2024 and is expected to reach USD 14.91 billion in 2025.
b. The global cardiac rhythm management devices market is expected to grow at a compound annual growth rate of 2.95% from 2025 to 2030 to reach USD 18.77 billion by 2030.
b. The defibrillator segment dominated the global CRM devices market and held the largest revenue share of 23.2% in 2024.
b. North America dominated the CRM devices market with a share of 49.8% in 2024. This is attributable increasing incidence of heart failures and arrhythmia and the availability of technologically advanced CRM devices.
b. Some key players operating in the CRM devices market include Medtronic, Jude Medical (Abbott); Boston Scientific Corporation; Koninklijke Philips N.V. Key; Physio-Control, Inc. (Stryker); Schiller; Zoll Medical Corporation; and BIOTRONIK.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










