- Home
- »
- Pharmaceuticals
- »
-
Cervical Cancer Treatment Market Size & Share Report 2025GVR Report cover
![Cervical Cancer Treatment Market Size, Share & Trends Report]()
Cervical Cancer Treatment Market Size, Share & Trends Analysis Report By Type (SCC, Adenocarcinoma), By Product (Prevention, Treatment), By Distribution Channel (Online, Hospital Pharmacies), And Segment Forecasts, 2018 - 2025
- Report ID: GVR-2-68038-718-6
- Number of Pages: 83
- Format: Electronic (PDF)
- Historical Range: 2013 - 2016
- Industry: Healthcare
Report Overview
The global cervical cancer treatment market size was valued at USD 6.3 billion in 2017 and is anticipated to grow at a compound annual growth rate (CAGR) of 5.0% from 2018 to 2025. Increased disease prevalence, proactive government initiatives, and advent of targeted therapies are the major factors contributing to the market expansion. Cervical cancer has a high mortality rate (nearly 50%), which can be reduced by diagnosis and prevention.
Since the disease progression is generally slow, precancerous changes provide possibilities for prevention and treatment. However, most cases are presented at later stages of disease progression due to lack of awareness or inaccessibility to diagnostic facilities. Human Papillomavirus (HPV) is a major causative factor for cervical cancer, generally acquired through sexual transmission. There are more than 100 strains of HPV, out of which 13 are high-risk or cancerous.
High vs low risk HPV strains are determined by the activated gene - E6 or E7. E6 has affinity for p53, leading to proteolytic degradation. On the other hand, E7 attaches to retinoblastoma; the binding displaces previously bound transcription factors and results in halting cell cycle and inhibiting apoptosis regulation. This disease can also be triggered by other factors, such as oral contraceptives, smoking, and HIV infection.
Lack of awareness about the diagnosis and treatment methods, high cost of treatment, and adverse effects associated with cancer therapy may hinder the market growth. Moreover, lack of targeted drugs with improved clinical profile at reduced costs and convenient administration schedule is also likely to obstruct market growth. However, rising R & D expenditure in oncologic diseases offer a promising future for the global cervical cancer treatment market in the foreseeable future.
Type Insights
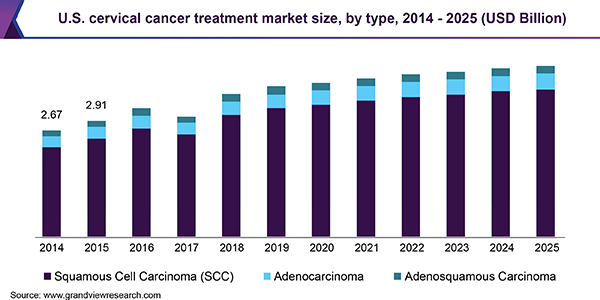
Squamous Cell Carcinoma (SCC) is the most prevalent type, accounting for more than 85% of all diagnosed cases. High-Grade Squamous Intraepithelial Lesion (HSIL) of the cervix may lead to SCC, if left untreated. Regular screening with Pap tests helps in the early detection and diagnosis of SCC. Certain ethnic races, such as Hispanic and African-American women, tend to be more prone to adenocarcinoma of the cervix. The differentiation between SCC and adenocarcinoma is based on the histology, as observed under the microscope. Cervical adenosquamous carcinoma is the rarest type, involving tissues that can be both squamous and glandular.
Product Insights
Products for cervical cancer can be broadly classified into prevention and treatment. Preventive options involve vaccines against HPV. Currently, there are two commercial vaccines - Gardasil and Cervarix. Treatment options involve biologics including Avastin and generics, such as topotecan, cisplatin, bleomycin, fluorouracil, paclitaxel, carboplatin, and doxorubicin.
Gardasil led the preventive vaccine segment in 2017, supported by strong commercial sales across the world. In addition, Gardasil 9 - an improvised, nonavalent version of Gardasil - was approved by the FDA in 2015. This label expansion gave Gardasil (quadrivalent) and Gardasil 9 (nonavalent) a distinct clinical advantage over Cervarix (bivalent). GlaxoSmithKline withdrew Cervarix from the U.S. market in 2016 due to low demand and intense competition.
Avastin (bevacizumab) was approved for the treatment of cervical cancer in 2014. This marked the arrival of biologics in this treatment landscape. Avastin was approved in Europe and Japan in 2015 and 2016, respectively. Following this, the FDA approved Keytruda (pembrolizumab) in 2018. Although generic products continue to capture significant market share, the arrival of biologics and other targeted therapies indicate a paradigm shift in this therapeutic space through the forecast period.
Distribution Channel Insights
Hospital pharmacies captured the largest share of the global market in 2017. Hospital pharmacies preserve the stock of products related to cervical cancer for out-patients as well as in-patients. The segment will be driven further by growing consumer awareness and increased investments in healthcare infrastructure in developing and underdeveloped countries.
Online pharmacies segment is expected to be the fastest-growing distribution channel segment. This growth can be attributed to user-friendly interfaces and convenience offered by these pharmacies, in terms of access, prices, and delivery. In addition, they have several discounts on purchase of a certain amount or offer bundle pricing for cancer products.
Regional Insights
North America led the global market in 2017. This was attributed to increased disease prevalence and consumer awareness along with technological advancements in the diagnosis and treatment of oncological diseases. The regional market is projected to expand further due to positive lifestyle changes. The Asia Pacific market is driven by improving economy and resultant emphasis on preventive healthcare.
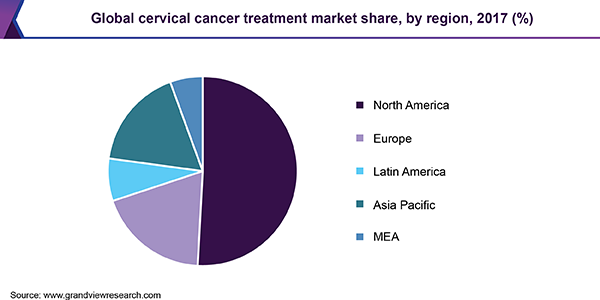
Rising number of health initiatives by private and government firms, adoption of better technologies, and growing consumer awareness are key drivers for the regional market. It is expected to witness steady growth over the forecast period on account of rising population, disease prevalence, and demand for cost-effective treatments. The trend of biosimilars in emerging markets, such as India and China, could potentially alter the therapeutic landscape in this region.
Key Companied & Market Share Insights
Some of the key companies in this market include F. Hoffmann-LA Roche AG; Merck & Co., Inc.; GlaxoSmithKline PLC; Allergan PLC; Pfizer, Inc.; Biocon Ltd.; Bristol-Myers Squibb; AstraZeneca PLC; and Eli Lilly & Co. Merck & Co. led the competitive space and is forecasted to continue holding the largest market share as a result of the strong commercial performances of Gardasil and Keytruda. In addition, Mvasi (Amgen/Allergan) and Bevacirel (Reliance/Lupin) have marked the emergence of biosimilars in this market.
Recent Developments
-
In June 2022, Roche introduced a Human papillomavirus (HPV) self-sampling solution that allows the patient to obtain the HPV screening sample privately at a healthcare institution under the instructions of a healthcare worker. The clinically-validated vaginal sample is examined on a Roche molecular instrument using the Roche cobas® HPV test.
-
In October 2021, Merck announced the approval of KEYTRUDA, Merck’s anti-PD-1 therapy, in combination with chemotherapy, with or without bevacizumab, by the U.S. Food and Drug Administration (FDA) for the treatment of patients with persistent, recurrent or metastatic cervical cancer whose tumors express PD-L1.
Cervical Cancer Treatment Market Report Scope
Report Attribute
Details
Revenue forecast in 2025
USD 10.6 billion
Growth rate
CAGR of 5.0% from 2018 to 2025
Base year for estimation
2017
Actual estimates/Historical data
2013 - 2016
Forecast period
2018 - 2025
Market representation
Revenue in USD Million and CAGR from 2018 to 2025
Regional scope
North America, Europe, Asia Pacific, Latin America, and MEA
Country scope
U.S., Canada, U.K., Germany, Japan, China, India, Brazil, Mexico, and South Africa
Report coverage
Revenue forecast, competitive landscape, growth factors, and trends
Customization scope
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Segments Covered in the ReportThis report forecasts revenue and volume growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2014 to 2025. For the purpose of this study, Grand View Research has segmented the global cervical cancer treatment market report on the basis of type, product, distribution channel, and region:
-
Type Outlook (Revenue, USD Million, 2014 - 2025)
-
Squamous Cell Carcinoma (SCC)
-
Adenocarcinoma
-
Adenosquamous Carcinoma
-
-
Product Outlook (Revenue, USD Million, 2014 - 2025)
-
Prevention
-
Gardasil/Gardasil9
-
Cervarix
-
-
Treatment
-
Avastin
-
Keytruda
-
Generics
-
Others
-
-
-
Distribution Channel Outlook (Revenue, USD Million, 2014 - 2025)
-
Hospital Pharmacies
-
Retail Pharmacies
-
Online Pharmacies
-
-
Regional Outlook (Revenue, USD Million, 2014 - 2025)
-
North America
-
The U.S.
-
Canada
-
-
Europe
-
The U.K.
-
Germany
-
-
Latin America
-
Brazil
-
Mexico
-
-
Asia Pacific
-
Japan
-
China
-
India
-
-
Middle East & Africa
-
South Africa
-
-
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





