- Home
- »
- Clinical Diagnostics
- »
-
COVID-19 Diagnostics Market Size & Share Report, 2030GVR Report cover
![COVID-19 Diagnostics Market Size, Share & Trends Report]()
COVID-19 Diagnostics Market Size, Share & Trends Analysis Report By Product & Service (Instruments, Reagents & Kits, Services), By Sample Type, By Test Type, By Mode, By End Use, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-4-68038-561-8
- Number of Report Pages: 150
- Format: PDF, Horizon Databook
- Historical Range: 2020 - 2023
- Forecast Period: 2021 - 2030
- Industry: Healthcare
COVID-19 Diagnostics Market Size & Trends
The global COVID-19 diagnostics market size was valued at USD 33.03 billion in 2023 and is expected to decline at a compound annual growth rate (CAGR) of 21.2% from 2024 to 2030. Certain factors such as rising government initiatives for COVID-19 testing and growing number of product launches are projected to accelerate the market growth during the forecast period. For instance, in September 2023, the government of U.S. awarded USD 600 million to accelerate the development and manufacturing of diagnostics tests. The government has allocated these funds to a number of research institutes and diagnostic tests manufacturers.
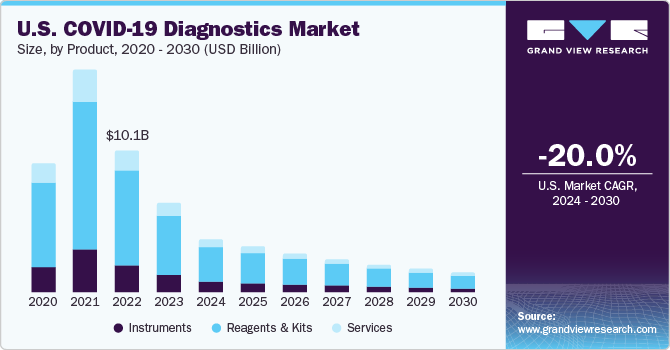
POC testing enables accurate real-time, lab-quality diagnostic results at patient care settings, such as urgent care centers, clinics, hospitals, and emergency rooms. In response to the pandemic, many diagnostic test developers are focusing on the development of fast and easy-to-use POC diagnostic tests to facilitate testing outside of laboratory settings. For instance, in March 2023, Lucira Health, Inc., launched its groundbreaking Lucira COVID-19 & Flu Home Test nationwide in the U.S. Additionally, this combination test has been included in Australian Register of Therapeutic Goods (ARTG), allowing healthcare professionals to use it in a point-of-care setting. Further, the health authorities are focusing on the development and supply of COVID-19 diagnostics tests, which in turn projected to have a positive impact on the market growth. In April 2021, the FDA approved an amended EUA request for various COVID-19 tests, expanding POC and OTC testing options.
Moreover, the agency mentioned that addition of PoC and OTC tests for screening will give workplaces, communities, schools, and other areas reliable & accurate options for serial screening tests. On September 23, 2020, Assure COVID-19 IgG/IgM Rapid Test Device secured reauthorization for its use as a POC antibody test for SARS-CoV-2 testing. The device was approved for emergency use by certain centers in July 2020 to aid identification of individuals with coronavirus prior to the onset of COVID-19 infection symptoms. Similarly, in September 2020, QIAGEN announced the launch of Access Anti-SARS-CoV-2 Antigen Test, a rapid portable antigen test that can provide result in less than 15 minutes and exhibits the potential to process 30 swab specimens per hour on an average. Thus, introduction of rapid POC tests has increased the efficiency as well as preference for such tests.
In addition, increase in funding and investments by various private & public organizations to boost the development of POC SARS-CoV-2 diagnostic tests is anticipated to support market growth. For instance, in March 2020, Mesa Biotech, Inc., a U.S.-based molecular diagnostic company, received USD 561,000 contract from the U.S. Department of Health and Human Services for development of a novel rapid molecular diagnostic test for COVID-19. Following this, in March 2020, Mesa Biotech received EUA for its new Accula SARS-CoV-2 Test, a POC test that gives results in minutes. On April 14, 2020, Mesa Biotech announced to ship around 10,000 Accula SARS-CoV-2 tests to enable testing outside of the clinical labs. Thus, approvals and launch of innovative POC diagnostic tests and increasing investments for development of these tests are anticipated to support market growth.
Market Concentration & Characteristics
Market growth stage is negative, and pace of the market growth is declining. The decline in COVID-19 diagnostics tests market could be attributed to factors such as decreasing infection rates, widespread vaccination efforts, and a shift in focus from diagnostic testing to preventive measures and treatments.
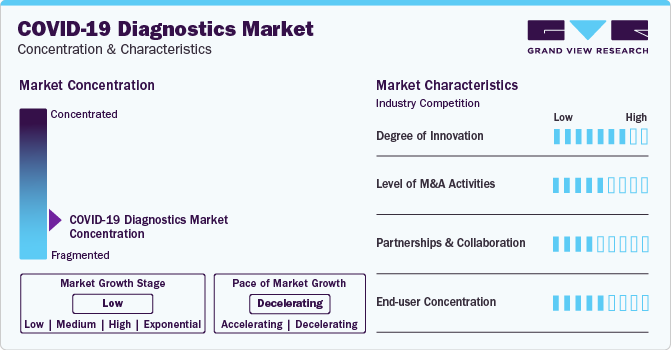
The SARS-CoV-2 diagnostics market is also characterized by a high level of merger and acquisition (M&A) activity by the leading players. Factors such as technology sharing and cost efficiency are playing a major role in high level of merger and acquisition (M&A) activity.
The COVID-19 diagnostics market is also witnessing considerable number of partnerships and collaborations. COVID-19 diagnostics test manufacturers are collaborating to leverage combined expertise, enhance production capabilities, and address global demand efficiently. Collaboration allows for the exchange of knowledge, resources, and technologies, accelerating the development and distribution of innovative testing solutions. Additionally, it helps meet the diverse needs of the market and navigate regulatory challenges more effectively.
Substantial end-user concentration in COVID-19 diagnostics market is likely due to centralized healthcare systems, where a limited number of healthcare providers or government agencies play a significant role in testing and decision-making.
Product & Service Insights
The services segment led the market and accounted for 47.7% of the revenue share in 2023. The surge in SARS-CoV-2 cases has proven advantageous for service providers, driven by the continuous rise in cases and an escalating demand for testing in recent years. These providers are expanding their technological capabilities by enhancing testing facilities in current labs, diagnostic centers, and launching new, high-capacity laboratories. Quest Diagnostics and Lab Corp are some of the key service providers operating in this market. These players are making ongoing attempts to keep up with the demand for testing, as the virus is spreading rapidly across the globe. For instance, in August 2020, Quest Diagnostics announced a reduction in the turnaround time for testing COVID-19 infection to 1 to 2 days.
Reagents and kits segment is projected to register substantial CAGR during the forecast period. The WHO has urged regions to ramp up early detection of disease, which consequently drives the adoption of reagents & kits on a large scale. Regulatory bodies have issued mass testing to combat the pandemic. Rising number of product approvals are projected to play considerable role in segment growth. For instance, in February 2021, Agilent announced launch of immunoassay kits for detection of COVID-19. However, some of the laboratories and hospitals are facing a shortage of reagents or kits required for large batches of testing due to disparities in supply chain and manufacturing. The CDC and FDA are continuously addressing challenges due to the shortage of reagents in the U.S. To overcome these challenges, FDA has granted Emergency Use Authorization (EUA) for several SARS-CoV-2 detection tests. This has allowed the relaxation of several stringent standards, enabling key market participants to quickly obtain FDA approval for their testing reagents and kits.
Test Type Insights
Molecular testing type segment held major market share in 2023. The utilization of RT-PCR technology in SARS-CoV-2 detection tests, measuring RNA levels in samples from exposed patients, is a key factor. The widespread adoption of PCR by the CDC, commercial labs, hospitals, and public health laboratories has notably boosted global market revenue. Additionally, the approvals for RT-PCR-based molecular diagnostic assays for early COVID-19 diagnosis contribute to the growth of this segment. Further, the market players are also taking robust efforts to introduce products in the market. For instance, in June 2022, F. Hoffmann-La Roche AG declared that the FDA approved EUA for its Cobas SARS-CoV-2 Duo for use on the Cobas 6800/8800 Systems.
The antibody testing segment is estimated to register considerable CAGR during the forecast period. Serology-based tests aid in monitoring the progress of the disease. In addition to blood serum or plasma samples, sputum, saliva, and other biological fluids are used for the detection of COVID-19 infection in individuals. Further, industrial developments are projected to offer positive environment for segment growth. For instance, in June 2022, Pictor Limited, a New Zealand-based biotech company, announced that it has achieved the CE mark for the company’s PictArray SARS-CoV-2 IgG enzyme-linked immunosorbent assay antibody test. This would further help determine whether the individual requires COVID-19 booster dose.
Sample Type Insights
Nasopharyngeal (NP) swab samples held major share in the market in 2023. This segment is expected to witness significant growth owing to the high demand for testing swabs due to increased awareness about COVID-19 testing and product availability. In addition, the introduction of various molecular diagnostic tests requiring nasopharyngeal swab samples is one of the factors contributing to the segment growth. The CDC and the U.S. FDA have recommended use of nasopharyngeal swabs as the preferred choice for swab-based diagnostic tests for the detection of SARS-CoV-2. The U.S. FDA has approved nasopharyngeal swabs manufactured by Copan Diagnostics, Puritan Medical Products, Quidel Corporation, BD, Thermo Fisher Scientific, and Hardy Diagnostics.
The blood sample testing segment is estimated to register considerable CAGR during the forecast period. With the introduction of blood-based serology tests for detection of SARS-CoV-2 infection, this segment has witnessed the fastest growth during pandemic. The potential of serology testing to identify preclinical signs of SARS-CoV-2 infection is a substantial advantage. Thus, the demand for blood-based antibody detection tests is significantly increasing in the market.
Mode Insights
The non-POC (centralized) testing segment dominated the market in 2023. Since the majority of SARS-CoV-2 tests are now performed in a lab setting, laboratory testing is currently the most adopted testing method in the market. The use of automated high throughput systems makes it possible to process many samples quickly and effectively without compromising the accuracy and reliability of the finished product. A successful COVID-19 response approach to stop the spread must include centralized testing as a key component. These factors make centralized testing an important element in a feasible COVID-19-response strategy to avoid the spread. Therefore, there is a growing demand for healthcare settings that offer decentralized services. For instance, in February 2023, Anavasi Diagnostics, which obtained the FDA's Emergency Use Authorization for their AscencioD COVID-19 Test and AscencioDx Molecular Detector. These products are portable and affordable, enabling high-quality point-of-care molecule testing in various settings, such as urgent care centers, mobile testing sites, and assisted nursing care centers.
Point-of-care testing mode segment is likely to register fastest CAGR during the study period. There is an urgent need for POC detection technologies that allow for decentralized, quick, accurate, and affordable diagnosis of COVID-19 infection. The field of COVID-19 POC diagnostics is quickly developing as a result of numerous technologies receiving commercialization approval in recent years. The first point-of-care diagnostic test for COVID-19 was approved by the U.S. FDA in March 2020 under EUA. Furthermore, several key market players are working on developing new products to achieve a significant market presence. For instance, in May 2022, LumiraDx announced that it has received a CE mark for its SARS-CoV-2 Ag Ultra test, which could provide results within 5 minutes.
End Use Insights
The laboratories end use segment dominated the market in 2023. The growth of segment is driven by various factors, including the increasing number of laboratories providing high-throughput technologies has significantly accelerated the efficiency of COVID-19 testing. For instance, in June 2020, the Indian Institute of Technology, Hyderabad, which, introduced an artificial intelligence-powered COVID-19 test designed to deliver fast and affordable testing solutions.
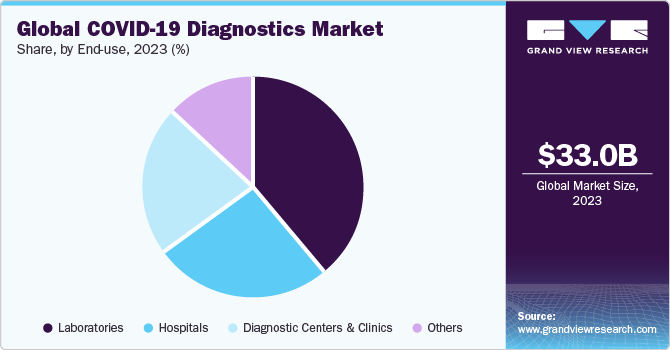
The diagnostic centers and clinics segment is expected to register a significant growth rate during the forecast period. According to NHS England, up to 20% of patients have hospital-acquired COVID-19 infection. This is encouraging many individuals to opt for diagnostics centers over hospitals for COVID-19 testing, which is a major factor contributing to revenue generation for this segment. Thus, the need to eliminate the risk of acquiring COVID-19 in hospitals is boosting the growth of the diagnostic centers and clinics segment.
Regional Insights
North America region dominated the market with a share of over 33.3% in 2023. The market growth is anticipated to be positively influenced over the forecast period due to various factors, including the widespread occurrence of COVID-19 infections and a well-established research and development infrastructure. Furthermore, the market growth is expected to be driven by an increase in regulatory approvals and the introduction of new diagnostic test kits and devices, which will enhance the availability of innovative solutions. This is likely to fuel the market expansion during the forecast period. For instance, in December 2022, Btnx's Rapid Response COVID-19 Antigen Self-test Kit received approval from the Government of Canada.
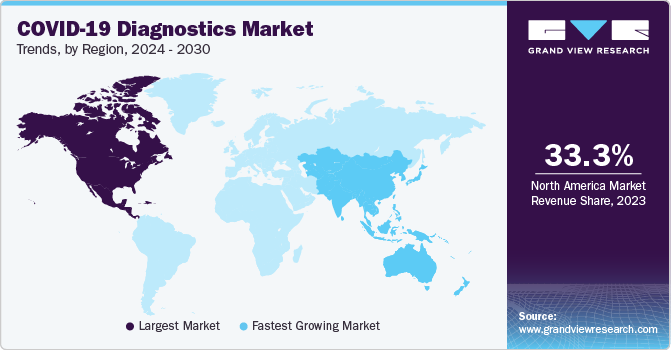
Asia Pacific is estimated to register fastest growth in the global COVID-19 diagnostics market during the forecast period. The increasing pool of local diagnostic reagents & kits manufacturers providing testing solutions for COVID-19 detection contributed to revenue generation in the region. Furthermore, collaborations between countries are expected to boost the adoption of COVID-19 diagnostic products in the region. Along with this, many companies in the region have entered into partnerships with international companies for developing and manufacturing COVID-19 diagnostic tests. In May 2020, Duke-NUS Medical School from Singapore collaborated with Diagnostics Development Hub & GenScript Biotech for the developing as well as manufacturing a COVID-19 detection system. India witnessed rapid growth in the global COVID-19 diagnostics market. This can be attributed to increasing testing capacity by government and new entrants in the market. The country has been expanding its testing capability for COVID-19.
COVID-19 Diagnostics Market Share Insights
Some of the key players operating in market include Hologic Inc., Thermo Fisher Scientific, Inc., F. Hoffman-La Roche Ltd., Perkin Elmer, Inc., and Abbott Laboratories. These companies focus on expanding their service portfolios, development of novel technologies, and strategic partnerships to strengthen their market presence.
In the COVID-19 testing market, emerging players are strategically involved in activities to strengthen their positions. These include research and development for innovative testing solutions, collaborations with healthcare entities for expanded reach, and the diversification of testing capabilities. By adapting to evolving market demands and technological advancements, these players aim to contribute significantly to the ongoing efforts in combating the COVID-19 pandemic.
Key COVID-19 Diagnostics Companies:
- Hologic Inc.
- Thermo Fisher Scientific, Inc.
- F. Hoffman-La Roche Ltd.
- Perkin Elmer, Inc.
- Veredus Laboratories
- 1drop Inc.
- ADT Biotech Sdn Bhd
- Laboratory Corporation of America Holdings
- bioMérieux SA
- Danaher
- Mylab Discovery Solutions Pvt. Ltd.
- Neuberg Diagnostics
- ALDATU BIOSCIENCES
- Quidel Corporation
- Quest Diagnostics
- altona Diagnostics GmbH
- Luminex Corporation
- Abbott
Recent Developments
-
In June 2022, a PerkinElmer company, EUROIMMUN, introduced CE-marked assays: Anti-SARS-CoV-2 Omicron ELISA (IgG) and Anti-SARS-CoV-2 RBD ChLIA (IgG). Both test systems detect IgG antibodies and are available in nations that accept the CE mark.
-
In May 2022, Neuberg declared that it plans to double its labs and touchpoints over the next two years. This initiative is expected to expand the company’s capacity in lab testing.
-
In November 2021, Thermo Fisher Scientific, Inc. confirmed that its PCR kit, TaqPath COVID-19 CE-IVD RT-PCR Kit, and TaqPath COVID-19 Combo Kit can detect the Omicron variant. This confirmation was expected positively impacted the company’s growth.
COVID-19 Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 20.31 billion
Revenue forecast in 2030
USD 6.25 billion
Growth Rate
CAGR of -21.2% from 2024 to 2030
Actual Years
2020 - 2023
Forecast period
2024 - 2030
Quantitative units
Revenue in USD billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
product & service, sample type, test type, mode, end-use, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S.; Canada; Germany; U.K.; France; Italy; Spain; Sweden; Denmark; Norway; China; India; Japan; South Korea; Australia; Thailand; Brazil; Mexico; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Hologic Inc.; Thermo Fisher Scientific, Inc.; F. Hoffman-La Roche Ltd.; Perkin Elmer, Inc.; Veredus Laboratories; 1drop Inc.; ADT Biotech SdnBhd; Laboratory Corporation of America Holdings; bioMérieux SA; Danaher; Mylab Discovery Solutions Pvt. Ltd.; Neuberg Diagnostics; ALDATU BIOSCIENCES; Quidel Corporation; Quest Diagnostics; altona Diagnostics GmbH; Luminex Corporation; Abbott
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global COVID-19 Diagnostics Market Report Segmentation
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2020 to 2030. For the purpose of this report, Grand View Research has segmented the global COVID-19 diagnostics market on the basis of product & service, sample type, test type, mode, end-use, and region.
-
Product & Service Outlook (Revenue, USD Million, 2020 - 2030)
-
Instruments
-
Reagents & Kits
-
Services
-
-
Sample Type Outlook (Revenue, USD Million, 2020 - 2030)
-
Nasopharyngeal (NP) swabs
-
Oropharyngeal (OP) swabs
-
Nasal swabs
-
Blood
-
Others
-
-
Test Type Outlook (Revenue, USD Million, 2020 - 2030)
-
Molecular (PCR) Testing
-
Antigen-based Testing
-
Antibody (Serology) Testing
-
Others
-
-
Mode Outlook (Revenue, USD Million, 2020 - 2030)
-
Point-of-Care (PoC)
-
Non-Point-of-Care (Non-PoC)
-
-
End-use Outlook (Revenue, USD Million, 2020 - 2030)
-
Laboratories
-
Hospitals
-
Diagnostic Centers and Clinics
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2020 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Norway
-
Denmark
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global COVID-19 diagnostics market is expected to grow at a compound annual growth rate of -21.2% from 2024 to 2030 to reach USD 6.25 billion by 2030.
b. The oropharyngeal & nasopharyngeal swabs segment is anticipated to lead the COVID-19 diagnostics market in 2023. This is attributable to the fact that nasopharyngeal swabs are the preferred choice for sample collection as recommended by the CDC.
b. Some key players in the COVID-19 diagnostics market are Thermo Fisher Scientific, Inc.; F. Hoffman-La Roche Ltd.; Perkin Elmer, Inc.; Neuberg Diagnostics; 1drop Inc.; Veredus Laboratories; ADT Biotech Sdn Bhd; altona Diagnostics GmbH; bioMérieux SA; Danaher; Mylab Discovery Solutions Pvt Ltd.; ALDATU BIOSCIENCES, and others.
b. Key factors driving the COVID-19 diagnostics market include the development of portable or an on-site diagnostic test for real-time management, robust WHO recommendations to make COVID-19 diagnostic testing a top priority, and integration of novel technologies & software solutions.
b. The global COVID-19 diagnostics market size is estimated at USD 33.03 billion in 2023 and is expected to reach USD 20.31 billion in 2024.
Table of Contents
Chapter 1. Methodology and Scope
1.1. Market Segmentation and Scope
1.2. Market Definitions
1.2.1. Information Analysis
1.2.2. Market Formulation & Data Visualization
1.2.3. Data Validation & Publishing
1.3. Research Assumptions
1.4. Information Procurement
1.4.1. Primary Research
1.5. Information or Data Analysis
1.6. Market Formulation & Validation
1.7. Market Model
1.8. Global Market: CAGR Calculation
1.9. Objective
1.9.1. Objective 1
1.9.2. Objective2
Chapter 2. Executive Summary
2.1. Market Snapshot
2.2. Segment Snapshot
2.3. Competitive Landscape Snapshot
Chapter 3. Market Variables, Trends, & Scope
3.1. Market Lineage Outlook
3.1.1. Parent Market Outlook
3.1.2. Related/Ancillary Market Outlook
3.2. Market Dynamics
3.2.1. Market Driver Analysis
3.2.1.1. Increase in the incidence of coronavirus globally
3.2.1.2. North America
3.2.1.3. Europe
3.2.1.4. Asia Pacific
3.2.1.5. Latin America
3.2.1.6. Middle East and Africa
3.2.1.7. Increasing product approvals by regulatory agencies
3.2.1.8. Government initiatives toward mass testing
3.2.1.9. Paradigm shift toward POC testing
3.2.2. Market Restraint Analysis
3.2.2.1. Limited testing capacity
3.2.2.2. Low demand due to supply ratio of COVID-19 reagents
3.2.3. Market opportunity analysis
3.2.3.1. Potential emerging markets
3.2.3.2. Favorable reimbursement scenario
3.2.3.3. Increasing penetration of telehealth in the diagnosis of Covid-19
3.2.4. Market Challenge analysis
3.2.4.1. Challenges pertaining to COVID-19 testing in healthcare settings
3.3. Industry Analysis Tools
3.3.1. Porter’s Five Forces Analysis
3.3.2. PESTEL Analysis
3.3.3. COVID-19 Diagnostics: Company Position Analysis
3.3.4. COVID-19 Diagnostics: Healthcare Spending and Funding Scenario in Different Countries
3.3.4.1. U.S.
3.3.4.2. China
3.3.4.3. Brazil
3.3.4.4. Germany
3.3.4.5. Others
3.3.5. Effect on Swabs Market
3.3.5.1. Production shortage for viral transport media
3.3.5.2. Companies leading the viral transport media market
3.3.5.3. COPAN Diagnostics
3.3.5.4. PURITAN MEDICAL PRODUCTS
3.3.5.5. Becton, Dickinson and Company (BD)
3.3.5.6. Thermo Fisher Scientific
3.3.5.7. Other emerging companies
3.3.6. Pricing Analysis
Chapter 4. Product & Service Business Analysis
4.1. Segment Dashboard
4.2. COVID-19 Diagnostics Market: Product & Service Movement Analysis, USD Million, 2023 & 2030
4.3. Reagents & Kits
4.3.1. Reagents & Kits Market, 2020 - 2030 (USD Million)
4.4. Instruments
4.4.1. Instruments Market, 2020 - 2030 (USD Million)
4.5. Services
4.5.1. Services Market, 2020 - 2030 (USD Million)
Chapter 5. Sample Type Business Analysis
5.1. Segment Dashboard
5.2. COVID-19 Diagnostics Market: Sample Type Movement Analysis, USD Million, 2023 & 2030
5.3. Nasopharyngeal (NP) Swabs
5.3.1. Nasopharyngeal (NP) Swabs Tests Market, 2020 - 2030 (USD Million)
5.4. Oropharyngeal (OP) Swabs
5.4.1. Oropharyngeal (OP) Swabs Market, 2020 - 2030 (USD Million)
5.5. Nasal Swabs
5.5.1. Nasal Swabs Market, 2020 - 2030 (USD Million)
5.6. Blood
5.6.1. Blood Market, 2020 - 2030 (USD Million)
5.7. Others
5.7.1. Others Market, 2020 - 2030 (USD Million)
Chapter 6. Test Type Business Analysis
6.1. Segment Dashboard
6.2. COVID-19 Diagnostics Market: Test Type Movement Analysis, USD Million, 2023 & 2030
6.3. Molecular (PCR) Testing
6.3.1. Molecular (PCR) Testing Market, 2020 - 2030 (USD Million)
6.4. Antigen-based Testing
6.4.1. Antigen-based Testing Market, 2020 - 2030 (USD Million)
6.5. Antibody (Serology) Testing
6.5.1. Antibody (Serology) Testing Market, 2020 - 2030 (USD Million)
6.6. Others
6.6.1. Others Market, 2020 - 2030 (USD Million)
Chapter 7. Mode Business Analysis
7.1. Segment Dashboard
7.2. COVID-19 Diagnostics Market: Mode Movement Analysis, USD Million, 2023 & 2030
7.3. Point-of-Care (PoC) Testing
7.3.1. Point-of-Care (PoC) Testing Market, 2020 - 2030 (USD Million)
7.4. Non-Point-of-Care (Non-PoC) Testing
7.4.1. Non-Point-of-Care (Non-PoC) Testing Market, 2020 - 2030 (USD Million)
Chapter 8. End-use Business Analysis
8.1. Segment Dashboard
8.2. COVID-19 Diagnostics Market: End-use Movement Analysis, USD Million, 2023 & 2030
8.3. Laboratories
8.3.1. Laboratories Market, 2020 - 2030 (USD Million)
8.4. Hospitals
8.4.1. Hospitals Market, 2020 - 2030 (USD Million)
8.5. Diagnostic Centers and Clinics
8.5.1. Diagnostic Centers and Clinics Market, 2020 - 2030 (USD Million)
8.6. Others
8.6.1. Others Market, 2020 - 2030 (USD Million)
Chapter 9. Regional Business Analysis
9.1. COVID-19 Diagnostics Market Share By Region, 2023 & 2030
9.2. North America
9.2.1. SWOT Analysis
9.2.2. North America COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.2.3. U.S.
9.2.3.1. Key Country Dynamics
9.2.3.2. Competitive Scenario
9.2.3.3. Regulatory Framework
9.2.3.4. U.S. COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.2.4. Canada
9.2.4.1. Key Country Dynamics
9.2.4.2. Competitive Scenario
9.2.4.3. Regulatory Framework
9.2.4.4. Canada COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.3. Europe
9.3.1. SWOT Analysis
9.3.2. Europe COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.3.3. UK
9.3.3.1. Key Country Dynamics
9.3.3.2. Competitive Scenario
9.3.3.3. Regulatory Framework
9.3.3.4. UK COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.3.4. Germany
9.3.4.1. Key Country Dynamics
9.3.4.2. Competitive Scenario
9.3.4.3. Regulatory Framework
9.3.4.4. Germany COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.3.5. France
9.3.5.1. Key Country Dynamics
9.3.5.2. Competitive Scenario
9.3.5.3. Regulatory Framework
9.3.5.4. France COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.3.6. Italy
9.3.6.1. Key Country Dynamics
9.3.6.2. Competitive Scenario
9.3.6.3. Regulatory Framework
9.3.6.4. Italy COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.3.7. Spain
9.3.7.1. Key Country Dynamics
9.3.7.2. Competitive Scenario
9.3.7.3. Regulatory Framework
9.3.7.4. Spain COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.3.8. Sweden
9.3.8.1. Key Country Dynamics
9.3.8.2. Competitive Scenario
9.3.8.3. Regulatory Framework
9.3.8.4. Sweden COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.3.9. Norway
9.3.9.1. Key Country Dynamics
9.3.9.2. Competitive Scenario
9.3.9.3. Regulatory Framework
9.3.9.4. Norway COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.3.10. Denmark
9.3.10.1. Key Country Dynamics
9.3.10.2. Competitive Scenario
9.3.10.3. Regulatory Framework
9.3.10.4. Denmark COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.4. Asia Pacific
9.4.1. SWOT Analysis
9.4.2. Asia Pacific COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.4.3. Japan
9.4.3.1. Key Country Dynamics
9.4.3.2. Competitive Scenario
9.4.3.3. Regulatory Framework
9.4.3.4. Japan COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.4.4. China
9.4.4.1. Key Country Dynamics
9.4.4.2. Competitive Scenario
9.4.4.3. Regulatory Framework
9.4.4.4. China COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.4.5. India
9.4.5.1. Key Country Dynamics
9.4.5.2. Competitive Scenario
9.4.5.3. Regulatory Framework
9.4.5.4. India COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.4.6. Australia
9.4.6.1. Key Country Dynamics
9.4.6.2. Competitive Scenario
9.4.6.3. Regulatory Framework
9.4.6.4. Australia COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.4.7. Thailand
9.4.7.1. Key Country Dynamics
9.4.7.2. Competitive Scenario
9.4.7.3. Regulatory Framework
9.4.7.4. Thailand COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.4.8. South Korea
9.4.8.1. Key Country Dynamics
9.4.8.2. Competitive Scenario
9.4.8.3. Regulatory Framework
9.4.8.4. South Korea COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.5. Latin America
9.5.1. SWOT Analysis
9.5.2. Latin America COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.5.3. Brazil
9.5.3.1. Key Country Dynamics
9.5.3.2. Competitive Scenario
9.5.3.3. Regulatory Framework
9.5.3.4. Brazil COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.5.4. Mexico
9.5.4.1. Key Country Dynamics
9.5.4.2. Competitive Scenario
9.5.4.3. Regulatory Framework
9.5.4.4. Mexico COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.5.5. Argentina
9.5.5.1. Key Country Dynamics
9.5.5.2. Competitive Scenario
9.5.5.3. Regulatory Framework
9.5.5.4. Argentina COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.6. MEA
9.6.1. SWOT Analysis
9.6.2. MEA COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.6.3. South Africa
9.6.3.1. Key Country Dynamics
9.6.3.2. Competitive Scenario
9.6.3.3. Regulatory Framework
9.6.3.4. South Africa COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.6.4. Saudi Arabia
9.6.4.1. Key Country Dynamics
9.6.4.2. Competitive Scenario
9.6.4.3. Regulatory Framework
9.6.4.4. Saudi Arabia COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.6.5. UAE
9.6.5.1. Key Country Dynamics
9.6.5.2. Competitive Scenario
9.6.5.3. Regulatory Framework
9.6.5.4. UAE COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
9.6.6. Kuwait
9.6.6.1. Key Country Dynamics
9.6.6.2. Competitive Scenario
9.6.6.3. Regulatory Framework
9.6.6.4. Kuwait COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Chapter 10. Competitive Landscape
10.1. Company Categorization
10.2. Strategy Mapping
10.3. Company Market Share Analysis, 2023
10.4. Company Profiles/Listing
10.4.1. F. Hoffmann-La Roche AG
10.4.1.1. Overview
10.4.1.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.1.3. Product Benchmarking
10.4.1.4. Strategic Initiatives
10.4.2. Thermo Fisher Scientific, Inc.
10.4.2.1. Overview
10.4.2.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.2.3. Product Benchmarking
10.4.2.4. Strategic Initiatives
10.4.3. PerkinElmer, Inc.
10.4.3.1. Overview
10.4.3.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.3.3. Product Benchmarking
10.4.3.4. Strategic Initiatives
10.4.4. 1drop, Inc.
10.4.4.1. Overview
10.4.4.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.4.3. Product Benchmarking
10.4.4.4. Strategic Initiatives
10.4.5. Veredus Laboratories
10.4.5.1. Overview
10.4.5.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.5.3. Product Benchmarking
10.4.5.4. Strategic Initiatives
10.4.6. ADT Biotech SdnBhd
10.4.6.1. Overview
10.4.6.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.6.3. Product Benchmarking
10.4.6.4. Strategic Initiatives
10.4.7. altona Diagnostics GmbH
10.4.7.1. Overview
10.4.7.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.7.3. Product Benchmarking
10.4.7.4. Strategic Initiatives
10.4.8. bioMérieux SA
10.4.8.1. Overview
10.4.8.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.8.3. Product Benchmarking
10.4.8.4. Strategic Initiatives
10.4.9. Neuberg Diagnostics
10.4.9.1. Overview
10.4.9.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.9.3. Product Benchmarking
10.4.9.4. Strategic Initiatives
10.4.10. Abbott Laboratories
10.4.10.1. Overview
10.4.10.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.10.3. Product Benchmarking
10.4.10.4. Strategic Initiatives
10.4.11. Luminex Corporation
10.4.11.1. Overview
10.4.11.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.11.3. Product Benchmarking
10.4.11.4. Strategic Initiatives
10.4.12. Laboratory Corporation of America Holdings
10.4.12.1. Overview
10.4.12.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.12.3. Product Benchmarking
10.4.12.4. Strategic Initiatives
10.4.13. Hologic Inc.
10.4.13.1. Overview
10.4.13.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.13.3. Product Benchmarking
10.4.13.4. Strategic Initiatives
10.4.14. Quest Diagnostics, Inc.
10.4.14.1. Overview
10.4.14.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.14.3. Product Benchmarking
10.4.14.4. Strategic Initiatives
10.4.15. Quidel Corporation
10.4.15.1. Overview
10.4.15.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.15.3. Product Benchmarking
10.4.15.4. Strategic Initiatives
10.4.16. ALDATU BIOSCIENCES
10.4.16.1. Overview
10.4.16.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.16.3. Product Benchmarking
10.4.16.4. Strategic Initiatives
10.4.17. Mylab Discovery Solutions Pvt. Ltd.
10.4.17.1. Overview
10.4.17.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.17.3. Product Benchmarking
10.4.17.4. Strategic Initiatives
10.4.18. Danaher Corporation
10.4.18.1. Overview
10.4.18.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.18.3. Product Benchmarking
10.4.18.4. Strategic Initiatives
10.4.19. Cepheid
10.4.19.1. Overview
10.4.19.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.19.3. Product Benchmarking
10.4.19.4. Strategic Initiatives
10.4.20. Beckman Coulter, Inc.
10.4.20.1. Overview
10.4.20.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.20.3. Product Benchmarking
10.4.20.4. Strategic Initiatives
List of Tables
Table 1 List of Secondary Sources
Table 2 List of Abbreviation
Table 3 Incidence rate of COVID-19 in North America
Table 4 Incidence rate of COVID-19 in Europe
Table 5 Incidence rate of COVID-19 in Asia Pacific
Table 6 Incidence rate of COVID-19 in Latin America
Table 7 Incidence rate of COVID-19 in Middle East and Africa
Table 8 Specimen collection and storage for COVID-19 testing
Table 9 Different COVID-19 diagnostic tests based on different technologies
Table 10 Global COVID-19 Diagnostics Market, By region, 2020 - 2030 (USD Million)
Table 11 Global COVID-19 Diagnostics Market, By product & service, 2020 - 2030 (USD Million)
Table 12 Global COVID-19 Diagnostics Market, By sample type, 2020 - 2030 (USD Million)
Table 13 Global COVID-19 Diagnostics Market, By test type, 2020 - 2030 (USD Million)
Table 14 Global COVID-19 Diagnostics Market, By mode, 2020 - 2030 (USD Million)
Table 15 Global COVID-19 Diagnostics Market, By end use, 2020 - 2030 (USD Million)
Table 16 North America COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 17 North America COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 18 North America COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 19 North America COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 20 North America COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 21 U.S. COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 22 U.S. COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 23 U.S. COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 24 U.S. COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 25 U.S.. COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 26 Canada COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 27 Canada COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 28 Canada COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 29 Canada COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 30 Canada COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million).
Table 31 Europe America COVID-19 Diagnostics Market, by country, 2020-2030, (USD Million)
Table 32 Europe COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 33 Europe COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 34 Europe COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 35 Europe COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 36 Europe COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million))
Table 37 U.K. COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 38 U.K. COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 39 U.K. COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 40 U.K. COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 41 U.K. COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 42 Germany COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 43 Germany COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 44 Germany COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 45 Germany COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 46 Germany COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 47 France COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 48 France COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 49 France COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 50 France COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 51 France COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 52 Italy COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 53 Italy COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 54 Italy COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 55 Italy COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 56 Italy COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 57 Spain COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 58 Spain COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 59 Spain COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 60 Spain COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 61 Spain COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 62 Sweden COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 63 Sweden COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 64 Sweden COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 65 Sweden COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 66 Sweden COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 67 Norway COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 68 Norway COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 69 Norway COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 70 Norway COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 71 Norway COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 72 Denmark COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 73 Denmark COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 74 Denmark COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 75 Denmark COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 76 Denmark COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 77 Asia Pacific America COVID-19 Diagnostics Market, by country, 2020-2030, (USD Million)
Table 78 Asia Pacific COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 79 Asia Pacific COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 80 Asia Pacific COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 81 Asia Pacific COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 82 Asia Pacific COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 83 China COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 84 China COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 85 China COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 86 China COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 87 China COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 88 India COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 89 India COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 90 India COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 91 India COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 92 India COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 93 Japan COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 94 Japan COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 95 Japan COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 96 Japan COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 97 Japan COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 98 South Korea COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 99 South Korea COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 100 South Korea COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 101 South Korea COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 102 South Korea COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 103 Australia COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 104 Australia COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 105 Australia COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 106 Australia COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 107 Australia COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 108 Thailand COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 109 Thailand COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 110 Thailand COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 111 Thailand COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 112 Thailand COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 113 Latin America COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 114 Latin America COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 115 Latin America COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 116 Latin America COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 117 Latin America COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 118 Brazil COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 119 Brazil COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 120 Brazil COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 121 Brazil COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 122 Brazil COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 123 Mexico COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 124 Mexico COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 125 Mexico COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 126 Mexico COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 127 Mexico COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 128 Argentina COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 129 Argentina COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 130 Argentina COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 131 Argentina COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 132 Argentina COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 133 MEA COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 134 MEA COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 135 MEA COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 136 MEA COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 137 MEA COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 138 South Africa COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 139 South Africa COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 140 South Africa COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 141 South Africa COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 142 South Africa COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 143 Saudi Arabia COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 144 Saudi Arabia COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 145 Saudi Arabia COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 146 Saudi Arabia COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 147 Saudi Arabia COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 148 UAE COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 149 UAE COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 150 UAE COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 151 UAE COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 152 UAE COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
Table 153 Kuwait COVID-19 diagnostics market estimates and forecasts, by product & service, 2020 - 2030 (USD Million)
Table 154 Kuwait COVID-19 diagnostics market estimates and forecasts, by sample type, 2020 - 2030 (USD Million)
Table 155 Kuwait COVID-19 diagnostics market estimates and forecasts, by test type, 2020 - 2030 (USD Million)
Table 156 Kuwait COVID-19 diagnostics market estimates and forecasts, by mode, 2020 - 2030 (USD Million)
Table 157 Kuwait COVID-19 diagnostics market estimates and forecasts, by end-use, 2020 - 2030 (USD Million)
List of Figures
Fig. 1 Market Research Process
Fig. 2 Information Procurement
Fig. 3 Primary Research Pattern
Fig. 4 Market Research Approaches
Fig. 5 Value Chain-Based Sizing & Forecasting
Fig. 6 Market Formulation & Validation
Fig. 7 COVID-19 Diagnostics Market Segmentation
Fig. 8 Market Driver Relevance Analysis (Current & Future Impact)
Fig. 9 Distribution of cases, as of 16th July 2020
Fig. 10 Distribution of cases, as of 4th June 2020
Fig. 11 Total number of cases in North America (1st March 2020-1st June 2020)
Fig. 12 Total number of cases in Europe (1st March 2020-1st June 2020)
Fig. 13 Total number of cases in Asia Pacific (excluding Oceania) (1st March 2020-1st June 2020)
Fig. 14 Total number of cases in Latin American countries (1st March 2020-1st June 2020)
Fig. 15 Total number of cases in South Africa (1st March 2020-1st June 2020)
Fig. 16 Market Restraint Relevance Analysis (Current & Future Impact)
Fig. 17 Company Position Analysis
Fig. 18 Healthcare expenditure in China, 2018-2023
Fig. 19 Healthcare expenditure in Brazil, 2018-2023
Fig. 20 Market Opportunity Relevance Analysis (Current & Future Impact)
Fig. 21 Market Challenge Relevance Analysis (Current & Future Impact)
Fig. 22 Penetration & Growth Prospect Mapping
Fig. 23 Swot Analysis, By Factor (Political & Legal, Economic and Technological)
Fig. 24 Porter’s Five Forces Analysis
Fig. 25 COVID-19 diagnostics market: Product & service outlook and key takeaways
Fig. 26 COVID-19 diagnostics market: Product & service movement analysis
Fig. 27 Global COVID-19 diagnostics reagents & kits market, 2020 - 2030 (USD Million)
Fig. 28 Global COVID-19 diagnostics instruments market, 2020 - 2030 (USD Million)
Fig. 29 Global COVID-19 diagnostic services market, 2020 - 2030 (USD Million)
Fig. 30 COVID-19 diagnostics market: Sample type outlook and key takeaways
Fig. 31 COVID-19 diagnostics market: Sample type movement analysis
Fig. 32 Global COVID-19 diagnostics nasopharyngeal swabs market, 2020 - 2030 (USD Million)
Fig. 33 Global COVID-19 diagnostics oropharyngeal swabs market, 2020 - 2030 (USD Million)
Fig. 34 Global COVID-19 diagnostics nasal swabs market, 2020 - 2030 (USD Million)
Fig. 35 Global COVID-19 diagnostics blood market, 2020 - 2030 (USD Million)
Fig. 36 Global COVID-19 diagnostics other samples market, 2020 - 2030 (USD Million)
Fig. 37 COVID-19 diagnostics market: Test type outlook and key takeaways
Fig. 38 COVID-19 diagnostics market: Test type movement analysis
Fig. 39 Global COVID-19 molecular (PCR) testing market, 2020 - 2030 (USD Million)
Fig. 40 Global COVID-19 antigen-based testing market, 2020 - 2030 (USD Million)
Fig. 41 Global COVID-19 antibody (Serology) testing market, 2020 - 2030 (USD Million)
Fig. 42 Global COVID-19 diagnostics other tests market, 2020 - 2030 (USD Million)
Fig. 43 COVID-19 diagnostics market: Mode outlook and key takeaways
Fig. 44 COVID-19 diagnostics market: Mode movement analysis
Fig. 45 Global COVID-19 Point-of-Care (PoC) testing market, 2020 - 2030 (USD Million)
Fig. 46 Global COVID-19 non-Point-of-Care (Non-PoC) testing market, 2020 - 2030 (USD Million)
Fig. 47 COVID-19 diagnostics market: End-use outlook and key takeaways
Fig. 48 COVID-19 diagnostics market: End-use movement analysis
Fig. 49 Global laboratories-based COVID-19 diagnostics market, 2020 - 2030 (USD Million)
Fig. 50 Global hospitals-based COVID-19 diagnostics market, 2020 - 2030 (USD Million)
Fig. 51 Global diagnostic centers and clinics-based COVID-19 diagnostics market, 2020 - 2030 (USD Million)
Fig. 52 Global other end-uses-based COVID-19 diagnostics market, 2020 - 2030 (USD Million)
Fig. 53 Regional Marketplace: Key Takeaways
Fig. 54 Regional Outlook, 2023 & 2030
Fig. 55 Regional Market Dashboard
Fig. 56 Regional Market Place: Key Takeaways
Fig. 57 North America
Fig. 58 North America COVID-19 Diagnostics Market Estimates and Forecast, 2020 - 2030 (USD Million)
Fig. 59 U.S. Key Country Dynamics
Fig. 60 U.S. COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 61 Canada Key Country Dynamics
Fig. 62 Canada COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 63 Europe COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 64 UK Key Country Dynamics
Fig. 65 UK COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 66 Germany Key Country Dynamics
Fig. 67 Germany COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 68 France Key Country Dynamics
Fig. 69 France COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 70 Italy Key Country Dynamics
Fig. 71 Italy COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 72 Spain Key Country Dynamics
Fig. 73 Spain COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 74 Sweden Key Country Dynamics
Fig. 75 Sweden COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 76 Norway Key Country Dynamics
Fig. 77 Norway COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 78 Denmark Key Country Dynamics
Fig. 79 Denmark COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 80 Asia Pacific COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 81 Japan Key Country Dynamics
Fig. 82 Japan COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 83 China Key Country Dynamics
Fig. 84 China COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 85 India Key Country Dynamics
Fig. 86 India COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 87 Australia Key Country Dynamics
Fig. 88 Australia COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 89 Thailand Key Country Dynamics
Fig. 90 Thailand COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 91 South Korea Key Country Dynamics
Fig. 92 South Korea COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 93 Latin America COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 94 Brazil Key Country Dynamics
Fig. 95 Brazil America COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 96 Mexico Key Country Dynamics
Fig. 97 Mexico America COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 98 Argentina Key Country Dynamics
Fig. 99 Argentina America COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 100 Middle East and Africa America COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 101 South Africa Key Country Dynamics
Fig. 102 South Africa America COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 103 Saudi Arabia Key Country Dynamics
Fig. 104 Saudi Arabia America COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 105 UAE Key Country Dynamics
Fig. 106 UAE America COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 107 Kuwait Key Country Dynamics
Fig. 108 Kuwait America COVID-19 Diagnostics Market, 2020 - 2030 (USD Million)
Fig. 109 Strategy MappingWhat questions do you have? Get quick response from our industry experts. Request a Free ConsultationMarket Segmentation
- COVID-19 Diagnostics Product & Service Outlook (Revenue, USD Million, 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- COVID-19 Diagnostics Sample Type Outlook (Revenue, USD Million, 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- COVID-19 Diagnostics Test Type Outlook (Revenue, USD Million, 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- COVID-19 Diagnostics Mode Outlook (Revenue, USD Million, 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- COVID-19 Diagnostics End-use Outlook (Revenue, USD Million, 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- COVID-19 Diagnostics Market: Regional Outlook (Revenue, USD Million, 2020-2030)
- North America
- North America COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- North America COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- North America COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- North America COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- North America COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- U.S.
- U.S. COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- U.S. COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- U.S. COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- U.S. COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- U.S. COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- U.S. COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Canada
- Canada COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Canada COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Canada COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Canada COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Canada COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Canada COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- North America COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Europe
- Europe COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Europe COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Europe COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Europe COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Europe COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- UK
- UK COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- UK COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- UK COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- UK COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- UK COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- UK COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Germany
- Germany COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Germany COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Germany COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Germany COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Germany COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Germany COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- France
- France COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- France COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- France COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- France COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- France COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- France COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Italy
- Italy COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Italy COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Italy COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Italy COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Italy COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Italy COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Spain
- Spain COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Spain COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Spain COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Spain COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Spain COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Spain COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Sweden
- Sweden COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Sweden COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Sweden COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Sweden COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Sweden COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Sweden COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Norway
- Norway COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Norway COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Norway COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Norway COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Norway COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Norway COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Denmark
- Denmark COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Denmark COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Denmark COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Denmark COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Denmark COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Denmark COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Europe COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Asia Pacific
- Asia Pacific COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Asia Pacific COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Asia Pacific COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Asia Pacific COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Asia Pacific COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Japan
- Japan COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Japan COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Japan COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Japan COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Japan COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Japan COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- China
- China COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- China COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- China COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- China COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- China COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- China COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- India
- India COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- India COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- India COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- India COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- India COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- India COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Australia
- Australia COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Australia COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Australia COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Australia COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Australia COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Australia COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Thailand
- Thailand COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Thailand COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Thailand COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Thailand COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Thailand COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Thailand COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- South Korea
- South Korea COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- South Korea COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- South Korea COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- South Korea COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- South Korea COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- South Korea COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Asia Pacific COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Latin America
- Latin America COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Latin America COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Latin America COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Latin America COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Latin America COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Brazil
- Brazil COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Brazil COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Brazil COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Brazil COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Brazil COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Brazil COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Mexico
- Mexico COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Mexico COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Mexico COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Mexico COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Mexico COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Mexico COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Argentina
- Argentina COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Argentina COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Argentina COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Argentina COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Argentina COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Argentina COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Latin America COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- MEA
- MEA COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- MEA COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- MEA COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- MEA COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- MEA COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Saudi Arabia
- Saudi Arabia COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Saudi Arabia COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Saudi Arabia COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Saudi Arabia COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Saudi Arabia COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Saudi Arabia COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- South Africa
- South Africa COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- South Africa COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- South Africa COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- South Africa COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- South Africa COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- South Africa COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- UAE
- UAE COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- UAE COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- UAE COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- UAE COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- UAE COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- UAE COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Kuwait
- Kuwait COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- Instruments
- Reagents & Kits
- Services
- Kuwait COVID-19 Diagnostics Market, by Sample Type (Revenue, USD Million; 2020 - 2030)
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Blood
- Others
- Kuwait COVID-19 Diagnostics Market, by Test Type (Revenue, USD Million; 2020 - 2030)
- Molecular (PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
- Kuwait COVID-19 Diagnostics Market, by Mode (Revenue, USD Million; 2020 - 2030)
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
- Kuwait COVID-19 Diagnostics Market, by End Use (Revenue, USD Million; 2020 - 2030)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
- Kuwait COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- MEA COVID-19 Diagnostics Market, by Product & Service (Revenue, USD Million; 2020 - 2030)
- North America
COVID-19 Diagnostics Market Dynamics
Drivers: Increasing Product Approvals By Regulatory Agencies
The COVID-19 pandemic has spurred demand for diagnostic tests. Companies are intensifying R&D efforts to develop innovative tests and secure regulatory approvals. For example, Mammoth Biosciences received FDA Emergency Use Authorization (EUA) for its high-throughput CRISPR-based COVID-19 test in January 2022. Labcorp also received FDA approval for its nonprescription test that can detect various viruses including COVID-19 in May 2022. In addition, F. Hoffmann-La Roche AG received EUA from the FDA for its Elecsys Anti-SARS-CoV-2 antibody test in May 2020. India-based Mylab Discovery Solutions developed the first COVID-19 test kit and received approval from the Indian FDA/Central Drugs Standard Control Organization (CDSCO) in March 2020. Furthermore, strategic collaborations are underway to develop efficient COVID-19 diagnostic tests. For instance, the FDA approved EUA for the InspectIR COVID-19 Breathalyzer test in April 2022. Thermo Fisher Scientific is also developing a new COVID-19 serology test in collaboration with Mayo Clinic and WuXi Diagnostics. The increasing number of approved COVID-19 diagnostics is expected to significantly boost market growth.
Government Initiatives Toward Mass Testing
The COVID-19 pandemic has resulted in significant loss of life and other impacts. As of June 2022, WHO data shows approximately 537,591,764 confirmed cases and around 6,319,395 deaths globally. Countries like the U.S., Brazil, Russia, the UK, Spain, Italy, India, Germany, Peru, Turkey, Iran, and France have reported the most cases. Health organizations recommend mass testing as a strategy to control the disease’s spread. In response, governments are focusing on increasing testing rates. For example, Luxembourg initiated mass testing for 600,000 patients in May 2020 to prevent a second wave. The UK exceeded its target of 200,000 tests per day by the end of May 2020. Wuhan tested 11 million people in May 2020 after reporting 21 new cases. Hong Kong started voluntary mass testing in September 2020, testing about 1.78 million individuals. Despite criticism, the UK government announced plans for mass testing in September 2020, aiming to increase daily testing capacity to 500,000 by October end. The UK government also ordered 5.8 million POC testing kits in September 2020 and plans to test the entire population weekly from early 2021.
Restrains: Paradigm Shift Toward Poc Testing
Point-of-care (POC) testing provides real-time, lab-quality results at patient care sites like urgent care centers and hospitals. Amid the COVID-19 pandemic, diagnostic test developers are focusing on creating fast, user-friendly POC tests for non-laboratory settings. For example, Cepheid, a Danaher Corporation subsidiary, received FDA Emergency Use Authorization (EUA) for its POC COVID-19 test, Cepheid Xpert Xpress SARS-CoV-2, in March 2020. Similarly, Abbott Laboratories received EUA for its new molecular POC COVID-19 test that delivers results in less than 5 minutes. In April 2021, the FDA approved an amended EUA request for various COVID-19 tests, expanding POC and over-the-counter (OTC) testing options. The FDA-approved tests include Abbott BinaxNOW COVID-19 Antigen Self-Test and Quidel QuickVue At-Home OTC COVID-19 test for OTC at-home serial screening, BD Veritor System for Rapid Detection of SARS-CoV-2 for POC serial screening with a prescription, and Abbott BinaxNOW COVID-19 Ag 2 Card for POC serial screening without a prescription. Assure COVID-19 IgG/IgM Rapid Test Device received reauthorization for its use as a POC antibody test for SARS-CoV-2 testing in September 2020
What Does This Report Include?
This section will provide insights into the contents included in this COVID-19 diagnostics market report and help gain clarity on the structure of the report to assist readers in navigating smoothly.
COVID-19 diagnostics market qualitative analysis
-
Industry overview
-
Industry trends
-
Market drivers and restraints
-
Market size
-
Growth prospects
-
Porter’s analysis
-
PESTEL analysis
-
Key market opportunities prioritized
-
Competitive landscape
-
Company overview
-
Financial performance
-
Product benchmarking
-
Latest strategic developments
-
COVID-19 diagnostics market quantitative analysis
-
Market size, estimates, and forecast from 2018 to 2030
-
Market estimates and forecast for product segments up to 2030
-
Regional market size and forecast for product segments up to 2030
-
Market estimates and forecast for application segments up to 2030
-
Regional market size and forecast for application segments up to 2030
-
Company financial performance
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationResearch Methodology
A three-pronged approach was followed for deducing the COVID-19 diagnostics market estimates and forecasts. The process has three steps: information procurement, analysis, and validation. The whole process is cyclical, and steps repeat until the estimates are validated. The three steps are explained in detail below:
Information procurement: Information procurement is one of the most extensive and important stages in our research process, and quality data is critical for accurate analysis. We followed a multi-channel data collection process for COVID-19 diagnostics market to gather the most reliable and current information possible.
- We buy access to paid databases such as Hoover’s and Factiva for company financials, industry information, white papers, industry journals, SME journals, and more.
- We tap into Grand View’s proprietary database of data points and insights from active and archived monitoring and reporting.
- We conduct primary research with industry experts through questionnaires and one-on-one phone interviews.
- We pull from reliable secondary sources such as white papers and government statistics, published by organizations like WHO, NGOs, World Bank, etc., Key Opinion Leaders (KoL) publications, company filings, investor documents, and more.
- We purchase and review investor analyst reports, broker reports, academic commentary, government quotes, and wealth management publications for insightful third-party perspectives.
Analysis: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilized different methods of COVID-19 diagnostics market data depending on the type of information we’re trying to uncover in our research.
-
Market Research Efforts: Bottom-up Approach for estimating and forecasting demand size and opportunity, top-down Approach for new product forecasting and penetration, and combined approach of both Bottom-up and Top-down for full coverage analysis.
-
Value-Chain-Based Sizing & Forecasting: Supply-side estimates for understanding potential revenue through competitive benchmarking, forecasting, and penetration modeling.
-
Demand-side estimates for identifying parent and ancillary markets, segment modeling, and heuristic forecasting.
-
Qualitative Functional Deployment (QFD) Modelling for market share assessment.
Market formulation and validation: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilize different methods of data analysis depending on the type of information we’re trying to uncover in our research.
-
Market Formulation: This step involves the finalization of market numbers. This step on an internal level is designed to manage outputs from the Data Analysis step.
-
Data Normalization: The final market estimates and forecasts are then aligned and sent to industry experts, in-panel quality control managers for validation.
-
This step also entails the finalization of the report scope and data representation pattern.
-
Validation: The process entails multiple levels of validation. All these steps run in parallel, and the study is forwarded for publishing only if all three levels render validated results.
COVID-19 Diagnostics Market Categorization:
The COVID-19 diagnostics market was categorized into six segments, namely product & service (Instruments, Reagents & Kits, Services), sample type (Nasopharyngeal swabs, Oropharyngeal swabs, Nasal swabs, Blood), test type (Molecular, Antigen-based, Antibody Testing), mode (Point-of-Care, Non-Point-of-Care), end-use (Laboratories, Hospitals, Diagnostic Centers and Clinics), and regions (North America, Europe, Asia Pacific, Latin America, and Middle East Africa)
Segment Market Methodology:
The COVID-19 diagnostics market was segmented into product & service, sample type, test type, mode, end-use, and regions. The demand at a segment level was deduced using a funnel method. Concepts like the TAM, SAM, SOM, etc., were put into practice to understand the demand. We at GVR deploy three methods to deduce market estimates and determine forecasts. These methods are explained below:
Market research approaches: Bottom-up
-
Demand estimation of each product across countries/regions summed up to from the total market.
-
Variable analysis for demand forecast.
-
Demand estimation via analyzing paid database, and company financials either via annual reports or paid database.
-
Primary interviews for data revalidation and insight collection.
Market research approaches: Top-down
-
Used extensively for new product forecasting or analyzing penetration levels.
-
Tool used invoice product flow and penetration models Use of regression multi-variant analysis for forecasting Involves extensive use of paid and public databases.
-
Primary interviews and vendor-based primary research for variable impact analysis.
Market research approaches: Combined
- This is the most common method. We apply concepts from both the top-down and bottom-up approaches to arrive at a viable conclusion.
Regional Market Methodology:
The COVID-19 diagnostics market was analyzed at a regional level. The globe was divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, keeping in focus variables like consumption patterns, export-import regulations, consumer expectations, etc. These regions were further divided into seventeen countries, namely, the U.S.; Canada; Germany; the UK; France; Spain; Italy; Russia; China; India; Japan; South Korea; Australia; Brazil; Mexico; South Africa; Saudi Arabia.
All three above-mentioned market research methodologies were applied to arrive at regional-level conclusions. The regions were then summed up to form the global market.
COVID-19 diagnostics market companies & financials:
The COVID-19 diagnostics market was analyzed via companies operating in the sector. Analyzing these companies and cross-referencing them to the demand equation helped us validate our assumptions and conclusions. Key market players analyzed include:
-
F. HOFFMANN-LA ROCHE AG - F. Hoffmann-La Roche AG is a global healthcare corporation that functions in two divisions: pharmaceuticals and diagnostics. The firm is involved in the research, development, and production of molecular diagnostic reagents, testing kits, and systems. It also offers automated tools, software, IT solutions, and consumables that are widely used in in vitro research and diagnostics. The company serves a variety of clients, including hospitals, private medical labs, research labs, patients, and doctors. At present, it operates under the umbrella of Roche Holding AG and has a presence in over 100 countries.
-
THERMO FISHER SCIENTIFIC, INC. - Thermo Fisher Scientific, Inc. is a creator and provider of analytical tools, devices, consumables, reagents, software, and services for a range of uses, such as diagnostics, research, manufacturing, and drug discovery. The firm runs through four key divisions: Analytical Instruments, Specialty Diagnostics, Life Sciences Solutions, and Laboratory Products & Services. The Specialty Diagnostics division, offers diagnostic reagents, testing kits, instruments, culture media, and related products to cater to clients in healthcare, clinical, pharmaceutical, industrial, and food safety labs.
-
PERKINELMER, INC. - PerkinElmer, Inc. offers solutions, services, and technologies to various markets such as research, industrial, environmental, diagnostics, and laboratory services. The company’s range of products includes tools for genetic testing & diagnostics, analytical instruments, medical imaging products, software, consumables, and other instruments. It serves a wide array of sectors, including chemicals, consumer manufacturing, energy, environmental analysis, healthcare, food beverage & nutraceuticals, forensics, and life sciences. Additionally, it offers services like automation integration, contract research services, and reagent & instrument services to a variety of end-use industries. The company has a presence in over 150 countries. EUROIMMUN, a subsidiary of PerkinElmer, Inc. based in Germany, is engaged in the creation of COVID-19 testing solutions.
-
1DROP, INC. - 1drop, Inc. is a mobile healthcare startup that concentrates on creating innovative mobile solutions for the prevention and control of various diseases. The company originated as a technological offshoot from the C-Lab program of Samsung Electronics. It has over five products in its portfolio that have been approved for use in key markets, including Europe and the U.S.
-
VEREDUS LABORATORIES PTE LTD. - Veredus Laboratories Pte Ltd., a medical device firm, excels in the creation, development, and promotion of unique multiplexed molecular solutions. The firm serves various markets, including infectious diseases, food testing, custom testing, and biosurveillance. In 2018, it became a part of Sekisui Chemical Co., Ltd., a Japanese company known for manufacturing high-performance materials. Since the acquisition, Veredus Laboratories has been functioning as a wholly-owned subsidiary of Sekisui Chemical Co., Ltd.
-
ADT BIOTECH SDN BHD - ADT Biotech SDN BHD, a medical device enterprise based in Malaysia, is engaged in the creation and production of diagnostic tests and technologies. The firm offers swift and precise diagnostic solutions to healthcare providers, facilitating improved treatment choices and patient management. In 2013, the company broadened its reach by setting up its first subsidiary, ADT India, in New Delhi. Further expansion occurred in 2017 with the establishment of ADT Korea in Seoul.
-
ALTONA DIAGNOSTICS GMBH - Altona Diagnostics GmbH, a medical device firm, specializes in the research, development, and marketing of molecular diagnostic tests for identifying infectious diseases. The company holds an ISO 13485 certification and adheres to GMP guidelines in manufacturing its products. It has expanded its global presence with subsidiaries in Great Britain, France, Canada, the U.S., Italy, Brazil, India, and Argentina.
-
BIOMÉRIEUX SA - bioMérieux SA, a global biotechnology firm, is renowned for its expertise in the area of in vitro diagnostics (IVD) for both medical and industrial applications. The company is involved in the design, development, and production of diagnostic systems, offering a range of solutions including reagents, instruments, and software. Its products are extensively used for the diagnosis of infectious diseases, cardiovascular disorder testing, and cancer screening and monitoring. At present, bioMérieux SA operates in over 150 countries, thanks to its distribution network and 42 subsidiaries.
-
NEUBERG DIAGNOSTICS - Neuberg Diagnostics, a network of laboratories spanning India, South Africa, and the UAE, offers an extensive array of diagnostic tests for a variety of disorders, including COVID-19. The Neuberg consortium was established by several founding members, namely Ehrlich Laboratory in Chennai, Anand Diagnostic Laboratory in Bangalore, Global Labs in South Africa, Supratech Micropath in Ahmedabad, and Minerva Diagnostics in Dubai. With a robust network in the Middle East and Africa, Neuberg Diagnostics is currently concentrating on broadening its footprint in the U.S. and Europe.
-
ABBOTT - Abbott, a healthcare corporation, operates in five main areas: pharmaceuticals, medical devices, nutrition, vascular care, and diagnostics, and has a presence in over 150 countries. In October 2011, Abbott revealed plans to split into two entities, one concentrating on research-oriented pharmaceuticals and the other on a variety of medical products. The acquisition of Alere, Inc. was completed by Abbott in September 2017, marking a significant expansion of its diagnostics division.
Value chain-based sizing & forecasting
Supply Side Estimates
-
Company revenue estimation via referring to annual reports, investor presentations, and Hoover’s.
-
Segment revenue determination via variable analysis and penetration modeling.
-
Competitive benchmarking to identify market leaders and their collective revenue shares.
-
Forecasting via analyzing commercialization rates, pipelines, market initiatives, distribution networks, etc.
Demand side estimates
-
Identifying parent markets and ancillary markets
-
Segment penetration analysis to obtain pertinent
-
revenue/volume
-
Heuristic forecasting with the help of subject matter experts
-
Forecasting via variable analysis
COVID-19 Diagnostics Market Report Objectives:
-
Understanding market dynamics (in terms of drivers, restraints, & opportunities) in the countries.
-
Understanding trends & variables in the individual countries & their impact on growth and using analytical tools to provide high-level insights into the market dynamics and the associated growth pattern.
-
Understanding market estimates and forecasts (with the base year as 2020, historic information 2021, and forecast from 2022 to 2030). Regional estimates & forecasts for each category are available and are summed up to form the global market estimates.
COVID-19 Diagnostics Market Report Assumptions:
-
The report provides market value for the base year 2020 and a yearly forecast till 2030 in terms of revenue/volume or both. The market for each of the segment outlooks has been provided on region & country basis for the above-mentioned forecast period.
-
The key industry dynamics, major technological trends, and application markets are evaluated to understand their impact on the demand for the forecast period. The growth rates were estimated using correlation, regression, and time-series analysis.
-
We have used the bottom-up approach for market sizing, analyzing key regional markets, dynamics, & trends for various products and end-users. The total market has been estimated by integrating the country markets.
-
All market estimates and forecasts have been validated through primary interviews with the key industry participants.
-
Inflation has not been accounted for to estimate and forecast the market.
-
Numbers may not add up due to rounding off.
-
Europe consists of EU-8, Central & Eastern Europe, along with the Commonwealth of Independent States (CIS).
-
Asia Pacific includes South Asia, East Asia, Southeast Asia, and Oceania (Australia & New Zealand).
-
Latin America includes Central American countries and the South American continent
-
Middle East includes Western Asia (as assigned by the UN Statistics Division) and the African continent.
Primary Research
GVR strives to procure the latest and unique information for reports directly from industry experts, which gives it a competitive edge. Quality is of utmost importance to us, therefore every year we focus on increasing our experts’ panel. Primary interviews are one of the critical steps in identifying recent market trends and scenarios. This process enables us to justify and validate our market estimates and forecasts to our clients. With more than 8,000 reports in our database, we have connected with some key opinion leaders across various domains, including healthcare, technology, consumer goods, and the chemical sector. Our process starts with identifying the right platform for a particular type of report, i.e., emails, LinkedIn, seminars, or telephonic conversation, as every report is unique and requires a differentiated approach.
We send out questionnaires to different experts from various regions/ countries, which is dependent on the following factors:
-
Report/Market scope: If the market study is global, we send questionnaires to industry experts across various regions, including North America, Europe, Asia Pacific, Latin America, and MEA.
-
Market Penetration: If the market is driven by technological advancements, population density, disease prevalence, or other factors, we identify experts and send out questionnaires based on region or country dominance.
The time to start receiving responses from industry experts varies based on how niche or well-penetrated the market is. Our reports include a detailed chapter on the KoL opinion section, which helps our clients understand the perspective of experts already in the market space.
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationShare this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





