- Home
- »
- Medical Devices
- »
-
Deep Brain Stimulation Devices Market, Industry Report 2030GVR Report cover
![Deep Brain Stimulation Devices Market Size, Share & Trends Report]()
Deep Brain Stimulation Devices Market (2025 - 2030) Size, Share & Trends Analysis Report By Product (Single Channel, Dual-Channel), By Application (Pain Management, Epilepsy, Essential Tremor), By End-use, By Region, And Segment Forecasts
- Report ID: 978-1-68038-335-5
- Number of Report Pages: 300
- Format: PDF
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Deep Brain Stimulation Devices Market Summary
The Global Deep Brain Stimulation Devices Market size was estimated at USD 1.40 billion in 2024 and is projected to reach USD 2.50 billion by 2030, growing at a CAGR of 10.2% from 2025 to 2030.The major factors driving the market growth are rising prevalence of neurological disorders such as Parkinson’s disease and epilepsy, introduction of technologically advanced products, escalating product demand as add-on therapy, and the growing demand for minimally invasive procedures.
Key Market Trends & Insights
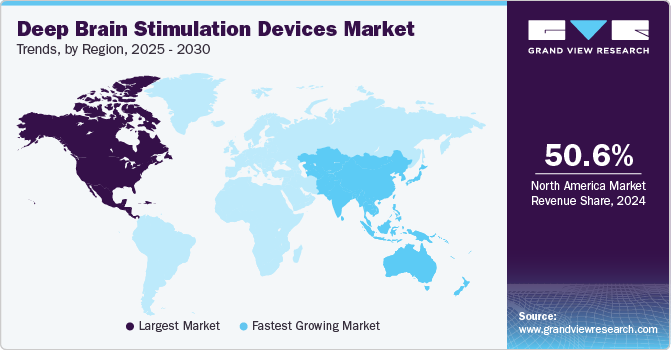
- North America deep brain stimulation devices market dominated the global market with a share of 50.55% in 2024.
- The deep brain stimulation devices market in U.S. is expected to dominate the market over the forecast period.
- Based on product, the dual-channel segment is dominating the market with a share of 56.9% in 2024.
- Based on end use, hospitals dominated the overall deep brain stimulation devices market growth with a share of 51.45% in 2024.
- Basis on application, Parkinson’s disease segment is dominating the market with a share of 64.98% in 2024.
Market Size & Forecast
- 2024 Market Size: USD 1.40 Billion
- 2030 Projected Market Size: USD 2.50 Billion
- CAGR (2025-2030): 10.2%
- North America: Largest market in 2024
The rising prevalence of neurological disorders and lifestyle-related disorders will further drive the market growth. For instance, according to the World Stroke Organization (WSO) global stroke fact sheet for 2022, there are over 12.2 million new stroke cases reported annually worldwide. Furthermore, this data reveals that globally, one in every four individuals aged 25 or older is expected to experience a stroke during their lifetime. These sobering statistics underscore the critical importance of addressing stroke prevention and treatment on a global scale. Thus, due to such factors market is expected to grow in near future.
Moreover, external funding for R&D, collaboration, FDA approval, and demand for minimally invasive surgeries are other major factors propelling the market growth. For instance, in April 2022, a significant trial was initiated at Southmead Hospital in Bristol, featuring a novel miniaturized deep brain stimulation (DBS) device known as the Picostim DBS system. The primary objective of this study is to mitigate complications and reduce the costs typically associated with DBS surgery by introducing and testing this innovative device. Moreover, the introduction of this new device holds the potential to streamline existing DBS surgery, making it not only quicker but also safer and more cost-effective. This promising development could ultimately broaden access to DBS therapy, benefiting a larger segment of individuals dealing with Parkinson's disease by making the treatment more accessible and affordable. Thus, due to such activities, the demand for deep brain stimulation devices will increase in near future.
Increasing prevalence of neurological disorders, such as Parkinson’s disease, essential tremor, dystonia, and epilepsy, is a high impact rendering driver for the deep brain stimulators market. According to WHO, neurological diseases contribute 6.3% to the global disease burden and are one of the major causes of mortality worldwide, resulting in 13.2% deaths in developed countries and 16.8% in low- and middle-income countries. High mortality and disease burden create clinical urgency for incorporation of long-term solutions such as deep brain stimulators. Furthermore, growing awareness about the massive neurological disease burden has raised the demand for deep brain stimulators as an alternative therapy. This is expected to drive market growth during the forecast period.
Furthermore, technological advancements in DBS technologies are anticipated to create growth opportunities in this market. These technological improvements include multi-target stimulation, robot-assisted implantation, improved microelectrode designs, rechargeable implantable pulse generators, and personalized directed programming. For instance, in January 2024, Abbott announced that it received approval from the U.S. Food and Drug Administration (FDA) to launch the Liberta RC DBS system. The system is world's smallest rechargeable DBS device with remote programming, to treat people with movement disorders. The Liberta RC DBS system also requires the fewest recharges of any FDA-approved DBS system, needing only 10 recharge sessions a year for most patients.
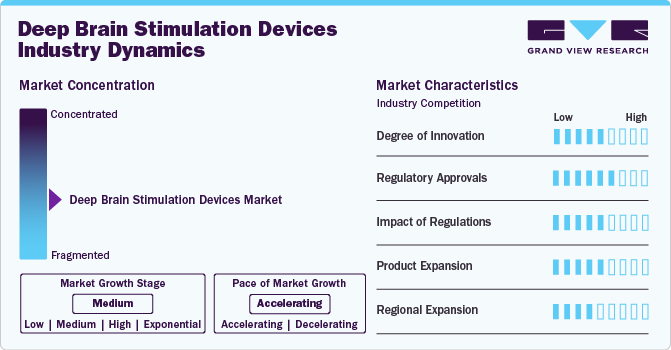
Market Concentration & Characteristics
The DBS Devices market is a highly competitive space with several established players and new entrants competing for market share. The deep brain stimulation devices industry is characterized by a high degree of growth owing to increasing prevalence of neurological disorders, technological advancements, and growing demand for minimally invasive treatments.
Key strategies implemented by players in the market are new product launches, expansion, acquisitions, partnerships, and other strategies. In January 2024, Abbott announced that it received approval from the U.S. Food and Drug Administration (FDA) to launch the Liberta RC DBS system, the world's smallest rechargeable DBS device with remote programming, to treat people living with movement disorders. The Liberta RC DBS system also requires the fewest recharges of any FDA-approved DBS system, needing only 10 recharge sessions a year for most people.
Several new technologies and products are being developed to improve patient outcomes, reduce costs, and increase adoption. For instance, in January 2024, Medtronic, one of the global leaders in healthcare technology, announced the U.S. Food and Drug Administration (FDA) approval of its Percept RC Deep Brain Stimulation (DBS) system. The rechargeable neurostimulator is the latest innovation in the Medtronic Percept family, which includes the Percept PC neurostimulator, BrainSense technology, and SenSight directional leads. This pioneering family of products is the only sensing-enabled DBS system on the market, empowering physicians to tailor treatment to individual patients with movement disorders such as Parkinson's disease, essential tremor, and dystonia, as well as epilepsy. Further, increased computing power is enabling the development of more sophisticated algorithms and simulations for optimizing DBS therapy.
Deep brain stimulation devices products are medical devices subject to strict regulatory standards. Regulatory bodies, such as U.S. FDA or CE (Conformité Européene) marking in Europe, set guidelines and standards to ensure the safety, effectiveness, and quality of these devices. Innovation in deep brain stimulation devices design, materials, and features often requires regulatory approvals. Manufacturers are required to navigate the regulatory landscape to ensure that new and improved products meet the necessary standards.
Regulatory bodies set quality and safety standards for medical equipment, including deep brain stimulation devices. Compliance with these standards is essential for manufacturers to ensure the safety of patients during transportation. Regulations require manufacturers to report adverse events associated with their devices, which can help identify potential safety issues and inform device improvements.
Product expansion in the deep brain stimulation devices industry involves the introduction of new products or the enhancement of existing ones to meet evolving patient needs, technological advancements, and market demands. Companies can introduce new product lines to address specific segments of the deep brain stimulation devices market. DBS devices are being explored for new indications, such as treating depression and obsessive-compulsive disorder.
The market exhibits elements of both fragmentation and consolidation, with the degree varying depending on factors such as product type, geographical region, and market segment. In certain segments of the deep brain stimulation devices industry, particularly in regions with a wide range of manufacturers and suppliers, the market can be fragmented. This fragmentation may be due to the presence of numerous small and medium-sized companies offering various deep brain stimulation devices products, including closed loop DBS devices, personalized DBS devices, ultrasound-based DBS devices, hybrid DBS devices, among others.
These companies often compete based on factors such as price, product differentiation, and distribution channels. Conversely, the market is also characterized by consolidation, particularly in segments dominated by a few large companies that hold significant market share. These companies may have established brand recognition, extensive distribution networks, and diverse product portfolios encompassing a range of Deep Brain Stimulation Devices solutions.
The market is influenced by various factors, including increasing demand for advanced treatments, growing demand for minimally invasive procedures, increasing prevalence of neurological disorders, and expanding indications for DBS devices. Regional expansion scenarios in the market are propelled by factors such as increasing awareness and education among healthcare professionals and patients about DBS devices, and growing government support and reimbursement for DBS devices.
Product Insights
Based on product, deep brain stimulation devices market is segmented into single-channel and dual-channel deep brain stimulation device. The dual-channel segment is dominating the market with a share of 56.9% in 2024 owing to its higher adoption in surgical procedures. Dual-channel devices represent some of the most secure and efficient tools employed in surgical interventions. Consequently, the surge in disabling neurological conditions' prevalence, the escalating demand for Parkinson’s disease(PD) surgeries, and the rising adoption of dual-channel DBS devices by a growing number of medical facilities have all contributed to the remarkable growth of this segment. Furthermore, the segment's expansion is being fueled by ongoing technological advancements and the introduction of new products.
However, single-channel deep brain stimulation device segment is expected to grow at the highest CAGR from 2025 to 2030. The consensus among healthcare experts is that single-channel DBS devices provide an extensive array of programming options for neurologists, consequently boosting their appeal and usage. Supporting this trend, the Parkinson's Foundation highlights the heightened vulnerability of the geriatric population to neurological ailments. As per the WHO report 2022, by 2050, the global population of individuals aged 60 and older will double to 2.1 billion, while those aged 80 and above will triple to reach 426 million by 2050, compared to 2020 figures. Given the increasing geriatric demographic, coupled with the rising patient awareness regarding neurological conditions, and the strong preference exhibited by healthcare professionals, the growth trajectory of this segment is expected to experience a significant upswing.
End-use Insights
In the end use segment, hospitals dominated the overall deep brain stimulation devices market growth with a share of 51.45% in 2024. The substantial growth witnessed in this segment can predominantly be attributed to the escalating number of DBS surgeries performed within hospital settings and the concurrent rise in the prevalence of conditions such as Parkinson's disease and essential tremor. In addition, the availability of technologically advanced DBS devices, coupled with favorable reimbursement policies, is poised to further propel the hospitals segment during the forecast period. A notable example of this is Medtronic, which offers comprehensive services aimed at securing and maintaining coverage, as well as facilitating payment for a range of DBS devices.
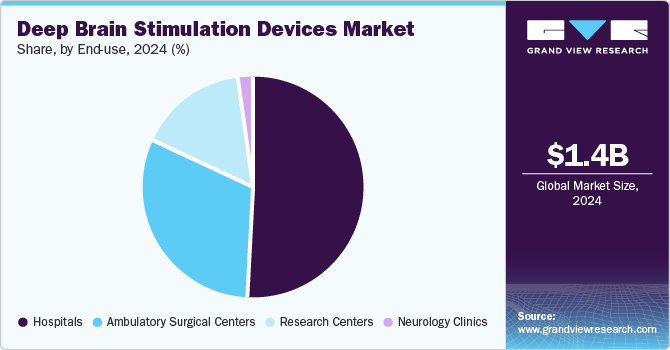
However, ASCs segment expected to grow at a highest CAGR from 2025 to 2030. This is due to lower cost of procedures as compared to hospitals, convenient access for patient care, reduced waiting time, and low infection rate as compared to neurology clinics and hospitals. Furthermore, according to a study conducted by Advancing Surgical Care, 92% of patients were satisfied with the medical care and service provided in the ASCs, thus boosting the segment growth. In addition, most of the neurosurgeries can now be performed at ASCs, as surgical procedures undertaken here are advanced and less invasive. Thus, growth of the segment can majorly be attributed to shorter procedure time and ongoing advancements in minimally invasive surgical technique.
Application Insights
On the basis of application, Parkinson’s disease segment is dominating the market with a share of 64.98% in 2024. This is due to increasing number of U.S. FDA approvals for deep brain stimulation therapies, high prevalence of PD worldwide, and increasing number of research and development activities. For instance, in March 2023, researchers at Michigan Technological University embarked on a groundbreaking initiative, leveraging neuromorphic computing to enhance both the efficacy and energy efficiency of deep brain stimulation systems utilized in the treatment of Parkinson's disease. This innovative approach is poised to significantly propel the growth of this segment during the forecast period.
However, epilepsy segment is expected to grow at the highest CAGR from 2025 to 2030. The increasing awareness of advanced epilepsy treatment options, combined with the ongoing development of healthcare infrastructure is expected to increase the growth of the segment in near future. As per the 2023 World Health Organization (WHO) report, approximately 50 million individuals are currently suffering with epilepsy, making it one of the most prevalent neurological disorders globally. Furthermore, it is noteworthy that nearly 80% of individuals with epilepsy reside in low- and middle-income countries.
Given the limitations of medication in addressing seizures and the less favorable success rates associated with surgical interventions, DBS has emerged as a valuable therapeutic approach in epilepsy management. This evolving landscape in epilepsy treatment is expected to significantly bolster the growth of this segment, offering promising prospects for improving the quality of life for those affected by this neurological condition.
Regional Insights
North America deep brain stimulation devices market dominated the global market with a share of 50.55% in 2024 owing to the increase in government funding and initiatives for raising awareness about PD is expected to drive the demand for deep brain stimulators. Furthermore, presence of major competitors, availability of sophisticated healthcare infrastructure, and supportive government initiatives are also responsible for the market growth in this region. For instance, the National Institute of Neurological Disorder and Stroke (NINDS) supports research activities related to DBS for determining its safety and effectiveness as the treatment of PD.
U.S. Deep Brain Stimulation Devices Market Trends
The deep brain stimulation devices market in U.S. is expected to dominate the market over the forecast period driven by the increasing prevalence of neurological disorders such as Parkinson's disease, dystonia, and essential tremors and growing number of clinical trials for treatment of psychiatric disorders. Advancements in DBS technology, such as enhancements in lead design, implantable pulse generators, and remote monitoring capabilities, are improving the effectiveness and safety of the DBS therapy in the U.S. According to 2022 Parkinson’s Foundation study, around one million people in the U.S. are living with Parkinson’s disease which is expected to reach nearly 1.2 million by 2030. Furthermore, high cost of DBS therapy and complexity and potential risks associated with DBS implantation is expected to hinder the growth of DBS devices market in the U.S.
Europe Deep Brain Stimulation Devices Market Trends
The deep brain stimulation devices market in Europe is expected to grow significantly over the forecast period. This growth can be attributed to the rising geriatric population and growing advancements in the deep brain stimulation devices market. Moreover, the region's advanced healthcare infrastructure, coupled with a focus on minimally invasive medical interventions, fosters the adoption of Deep Brain Stimulation Devices.
The deep brain stimulation devices market in the UK is expected to grow moderately over the forecast period.The increasing government spending on mental health services with about 14% of local NHS funding allocations is one of the primary driving factors for DBS devices market growth in UK. The NHS spent over USD 11.0 Billion in 2022/23 in England according to Mental Health Statistics report.
The France deep brain stimulation devices market is expected to grow over the forecast period owing to rising prevalence of essential tremor and Parkinson’s disease and growing government support for the clinical research on neurological disorders.
Deep brain stimulation devices market in Germany is witnessing a steady growth owing to increasing aging population and growing prevalence of neurological diseases. Germany is a significant market for DBS devices, with a large and growing population of patients suffering from neurological disorders. The country has a well-established healthcare system and a high demand for innovative and effective treatments.Further,increasing awareness of DBS therapy among patients and healthcare providers is driving the growth of the DBS devices market in Germany. DBS therapy is increasingly becoming popular as a treatment option for various neurological disorders, due to its high effectiveness. In addition, growing initiatives taken by German to promote the use of minimally invasive procedures, is also further boosting the demand for DBS devices.
Asia Pacific Deep Brain Stimulation Devices Market Trends
Asia Pacific is expected to be the fastest growing region over the forecast period. This is attributed to the rising prevalence of neurodegenerative disorders coupled with unmet demand for effective and long-term solutions. Rising awareness about neurological disease treatment options and improvements in clinical development framework of emerging economies are expected to drive the market growth in this region. Moreover, presence of high growth opportunities in developing countries such as Japan, China, and India are likely to contribute to market growth. Furthermore, establishment of organizations such as the Asia Pacific Centre for Neuromodulation (APCN), which is founded for conducting research and promoting awareness about associated benefits of deep brain stimulation, is anticipated to boost regional growth.
The deep brain stimulation devices market in China is expected to witness a significant growth over the forecast periodowing to the growing geriatric population, availability of innovative treatments, and increasing number of centers performing DBS surgeries which have heightened the demand for deep brain stimulation devices.
The deep brain stimulation devices market in India is propelled by factors such as the country’s growing healthcare sector, increasing awareness about neurological disorders, and rising demand for minimally invasive treatments have created a significant opportunity for the DBS devices market in India. The low cost of DBS therapy in India as compared to western countries such as U.S. and UK are also fostering the growth of DBS devices market in India. The total price of DBS therapy is 60-90% cheaper than in western countries.
The DBS devices market in Japan is witnessing a significant growth, driven by the increasing demand for innovative treatments for neurological disorders. In Japan, DBS is approved for the treatment of Parkinson's disease, and the market has been growing steadily over the past few years. The country's aging population, combined with the increasing incidence of neurological disorders, is driving the demand for DBS devices. According to the WHO data published in 2020, deaths due to Parkinson’s disease in Japan reached about 14,007 or 1.27% of total deaths. The Japanese Government is also implementing several initiatives to support the development of DBS devices in Japan which is expected to fuel the growth of DBS devices market during the forecast period.
Latin America Deep Brain Stimulation Devices Market Trends
The DBS devices market in Latin America is witnessing a significant growth, driven by increasing neurological disorders prevalence, technological advancements, and rising awareness of advanced therapeutic options. The Latin American healthcare landscape is undergoing transformation, marked by the expansion of healthcare infrastructure and improvements in medical technology. Countries such as Brazil and Argentina are at the forefront of this expansion, witnessing enhanced access to advanced neurological treatments, including DBS. The increasing prevalence of neurological disorders in these nations, exacerbated by urbanization and aging populations, propels the demand for effective treatment options. According to various health reports, disorders such as Parkinson's disease are anticipated to rise due to demographic shifts, thereby boosting the DBS market.
Middle East And Africa Deep Brain Stimulation Devices Market Trends
The DBS devices market in the Middle East and Africa is primarily driven by the increasing demand for innovative treatments for neurological disorders and high incidence of neurological disorders. The region is also home to a number of emerging economies, which are investing heavily in healthcare infrastructure which is creating a growth opportunity for DBS devices, as well as other medical devices and treatments in the region.
Saudi Arabia is a major hub for healthcare in the Middle East, with a growing population of nearly 36.9 million people in 2023 according to Worldometer data. The country has a well-established healthcare system, with a number of modern hospitals and medical facilities. The increasing prevalence of parkinson's disease, is driving demand for DBS devices. The Saudi Arabian government is implementing initiatives to promote the development of medical devices in the country. For example, the Saudi Arabian government has established a number of initiatives to support the development of medical devices in the country, including the "National Program for Medical Devices" and the "Saudi Arabian Medical Device Regulatory Authority".The Saudi Arabia market for medical equipment has an estimated value of over $2 billion and is growing annually at about 10%.Saudi Arabia is also pursuing privatization initiatives, Currently Saudi Arabian government spending accounts for over 60% of the country’s healthcare expenditure.
Turkey is expected to witness a significant growth over the forecast period, driven by increasing demand for innovative treatments for neurological disorders. The market is dominated by global players, however local companies are also emerging as key players. With advancements in technology and growing government support and funding for development of new medical devices, the Turkish DBS devices market is poised for continued growth.
Potential DBS Targets for Various Neurological Disorders under Clinical Trials, 2019 - 2023
Indication
Potential DBS Target
Trial
Tinnitus
Auditory pathways
Area LC
VIM
Phase I-II
Major depression
SCC
NAc
Habenula
Medial forebrain bundle
VC/VS
ITP
BNST
Phase I-III
Obsessive-compulsive disorder
NAc
STN
BNST
ITP
ALIC
VC/VS
Phase I-IV
Schizophrenia
Temporal cortex
NAc
VTA
SCC
Preclinical/Phase I
Addiction
NAc
STN
Phase I-III
Anorexia nervosa
SCC
NAc
ALIC
Phase I
Key Deep Brain Stimulation Devices Company Insights
The DBS devices market is highly competitive and moderately fragmented with a few established players namely Abbott, Boston Scientific Corporation, and Medtronic and several new entrants. Key players operating in DBS devices market are increasingly focusing on product launches, technological advancements, and other growth strategies, such as mergers & acquisitions to strengthen their foothold in the market. For instance, in July 2023, Boston Scientific Corporation announced FDA approval of its Vercise Neural Navigator 5 Software. This innovative software, when integrated with the Vercise Genus Deep Brain Stimulation (DBS) systems, is poised to provide clinicians with essential data for optimizing treatment for individuals living with Parkinson's disease or essential tremor. This advancement is set to enhance the precision and effectiveness of DBS therapy, benefiting patients dealing with these neurological conditions.
Key Deep Brain Stimulation Devices Companies:
The following are the leading companies in the deep brain stimulation devices market. These companies collectively hold the largest market share and dictate industry trends.
- Medtronic
- Boston Scientific Corporation
- Abbott
- Aleva Neurotherapeutics
- Newronika S.p.A.
- Beijing PINS Medical Co., Ltd.
Recent Developments
-
In September 2024, Abbott announced that it has launched a pivotal clinical trial named TRANSCEND study. This trial aims to assess the efficacy of the company’s DBS system in treating treatment-resistant depression (TRD), a challenging type of major depressive disorder.
-
In September 2024, Medtronic published the trial design and preliminary findings from the ADAPT-PD trial, which focuses on the adaptive DBS algorithm for personalized therapy in Parkinson’s disease.
-
In January 2024, Abbott received approval from the U.S. FDA to launch the Liberta RC DBS system which is world's smallest rechargeable DBS device with remote programming, to treat people living with movement disorders.
-
In January 2024, Medtronic received U.S. FDA approval of its Percept RC Deep Brain Stimulation (DBS) system. It is a rechargeable neurostimulator and is the latest innovation in the Medtronic Percept family, which includes the Percept PC neurostimulator, SenSight directional leads, and BrainSense technology.
-
In January 2021, Boston Scientific received U.S. FDA approval of its fourth-generation Vercise Genus DBS System. The portfolio, approved for conditional use in a magnetic resonance imaging(MRI) environment, consists of a family of Bluetooth-enabled, rechargeable and non-rechargeable, implantable pulse generators that power Cartesia Directional Leads, designed to provide optimal symptom relief.
Deep Brain Stimulation Devices Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 1.54 billion
Revenue forecast in 2030
USD 2.50 billion
Growth rate
CAGR of 10.2% from 2025 to 2030
Actual data
2018 - 2024
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, trends, and volume analysis
Segments covered
Product, application, end-use, region
Regional scope
North America; Europe; Asia Pacific; Central & South America; MEA
Country scope
U.S.; Canada; Mexico; UK ; Germany; France; Italy; Spain; Russia; Netherlands; Switzerland; Sweden; Ireland; Poland; Rest of Europe; Japan; China ; India ; Indonesia; South Korea; Thailand; Australia; Philippines; Malaysia; Singapore; Rest of Asia Pacific; Brazil ; Argentina; Colombia; Chile; Venezuela; Rest of Latin America; South Africa ; Saudi Arabia; UAE; Turkey; Iran; Rest of Middle East and Africa
Key companies profiled
Medtronic; Boston Scientific Corporation; Abbott; Aleva Neurotherapeutics; Newronika S.p.A.; Beijing PINS Medical Co., Ltd
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Deep Brain Stimulation Devices Market Report Segmentation
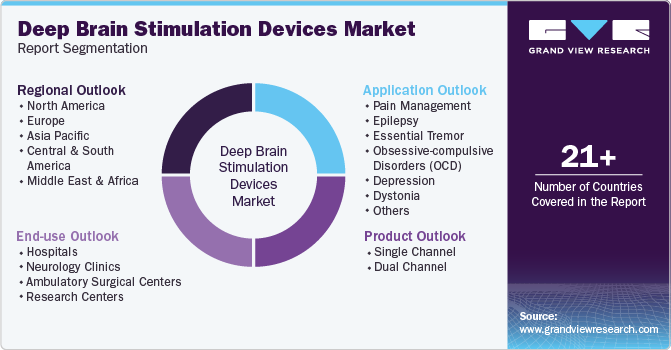
This report forecasts revenue growth at regional and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the deep brain stimulation devices market report based on product, application, end-use, and region:
-
Product Outlook (Revenue USD Million; 2018 - 2030)
-
Single Channel
-
Dual Channel
-
-
Application Outlook (Revenue USD Million; 2018 - 2030)
-
Pain Management
-
Epilepsy
-
Essential Tremor
-
Obsessive-compulsive Disorders (OCD)
-
Depression
-
Dystonia
-
Parkinson's Disease
-
Others
-
-
End-use Outlook (Revenue USD Million; 2018 - 2030)
-
Hospitals
-
Neurology Clinics
-
Ambulatory Surgical Centers
-
Research Centers
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Russia
-
Netherlands
-
Switzerland
-
Sweden
-
Ireland
-
Poland
-
Rest of Europe
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Indonesia
-
South Korea
-
Thailand
-
Australia
-
Philippines
-
Malaysia
-
Singapore
-
Rest of Asia Pacific
-
-
Central & South America
-
Brazil
-
Argentina
-
Colombia
-
Chile
-
Venezuela
-
Rest of Latin America
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Turkey
-
Iran
-
Rest of Middle East and Africa
-
-
Frequently Asked Questions About This Report
b. The global deep brain stimulation devices market size was estimated at USD 1.40 billion in 2024 and is expected to reach USD 1.54 billion in 2025.
b. The global deep brain stimulation devices market is expected to grow at a compound annual growth rate of 10.2% from 2025 to 2030 to reach USD 2.50 billion by 2030.
b. North America dominated the deep brain stimulation devices market with a share of 50.55% in 2024. This is attributable to the rising prevalence of movement and psychiatric disorders coupled with the increasing geriatric population in the region.
b. Some of the key players operating in the deep brain stimulation devices market include Medtronic, Boston Scientific Corporation, Abbott, and Aleva Neurotherapeutics
b. Key factors that are driving the deep brain stimulation devices market growth include the increasing incidence of neurological diseases, preference for a targeted approach, and technological advancements.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










