- Home
- »
- Biotechnology
- »
-
Dendritic Cell Cancer Vaccine Market Size & Share Report 2030GVR Report cover
![Dendritic Cell Cancer Vaccine Market Size, Share & Trends Report]()
Dendritic Cell Cancer Vaccine Market Size, Share & Trends Analysis Report By Products (CreaVax, Sipuleucel-T), By End-use (Pediatrics, Adults), By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-004-3
- Number of Report Pages: 200
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Report Overview
The global dendritic cell cancer vaccine market size was valued at USD 593.30 million in 2022 and is projected to expand at a compound annual growth rate (CAGR) of 12.18% from 2023 to 2030. The growth of the market is attributed to factors such as the increasing clinical trial activity related to cancer vaccines, growing demand for mRNA-based vaccines along with evolving cancer therapeutics. The growing demand for effective and personalized therapeutics is positively impacting the growth of the dendritic cell cancer vaccine market.
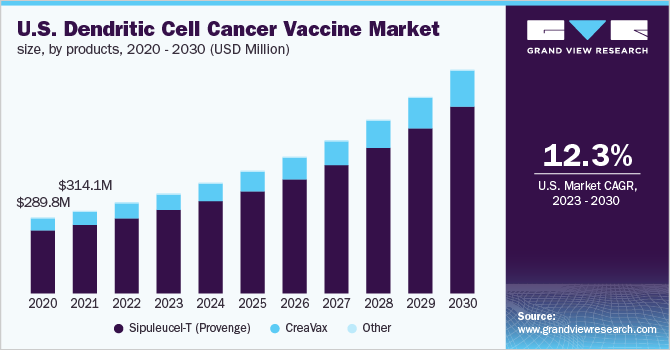
Furthermore, the rise in number of cancer patients, increased government funding for the development of cancer vaccines, and advancements in vaccine technology are driving the market growth. According to a report published by the International Agency for Research on Cancer in December 2020, 1 in 5 individuals worldwide will develop cancer in their lifetimes, and 1 in 8 men and 1 in 11 women die from malignancies. The same source stated that there would be 10.3 million deaths and 19.3 million new instances of the disease in 2020. The prevention of carcinoma is one of the most important challenges in front of this increasing burden. As biological response modifiers, vaccines are essential for the maintenance of the immune system.
Among all the currently available immunotherapy strategies, dendritic cell vaccines are the most potent antigen-presenting cells for the effective proliferation of T-cells. The specificity of the treatment has boosted its range of applications and is also witnessing global acceptance. As a result, several companies are collaborating to introduce a novel product. For instance, in March 2022, BioNTech signed into a collaboration with Medigene by which Medigene received USD 29 million as an upfront research fund for the co-development of immunotherapies focusing on multiple types of carcinoma. Further, BioNTech will utilize Medigene’s discovery platform and successfully acquire a preclinical asset.
Immunotherapy has great potential for treating minimal residual disease (MRD) by the use of dendritic cells and many studies have yielded promising results with no signs of recurrence of tumor growth. For instance, in May 2022, researchers of the AML-VACCiN consortium, clinically developed a dendritic cell vaccine- DCP-001, so as to vaccinate patients with acute myeloid leukemia to eradicate MRD and to effectively reduce the risk of a relapse. The vaccine candidate is designated as an orphan medicinal product in the EU after reliable results from trial phases I & II.
Product Insights
The Sipuleucel-T (Provenge) segment held the largest share in 2022. The ability of Sipuleucel-T to effectively enhance an individual’s immunity against tumor cells is increasing its market demand. The vaccine was approved for usage in 2010 by the U.S. FDA for the treatment of castrate-resistant prostate carcinoma. Many studies have shown positive inferences by using Sipuleucel-T in terms of immunity, minimal side effects, and an increase in the lifespan of a survivor. For instance, in October 2020, Dendreon Pharmaceuticals declared the publication of an analysis that examined the real-world survival outcomes in men with metastatic castrate-resistant prostate carcinoma on treatment with Sipuleucel-T and other associative oral medications.
The CreaVax is anticipated to grow significantly during the forecast period. It is an autologous dendritic cell-based cancer vaccine is created from peripheral blood mononuclear cells (PBMC) of the patient and sensitized with tumor lysate and KLH before developing into dendritic cells (Keyhole Limpet Hemocyanin). Creavax is a therapeutic vaccine for renal cell carcinoma produced by Creagene in 2007. CreaVax is currently under Phase III clinical trial investigation for its utility in the treatment of hepatocellular carcinoma.
End-use Insights
The adult vaccines segment held the highest market share in 2022 owing to the rising incidence of cancers across the globe. Moreover, many researchers across the globe are carrying out extensive research for the development of effective vaccine candidates to achieve potential therapeutic strategies. For instance, in March 2022, Maryland-based Northwest Biotherapeutics declares the initiation of the production of the first personalized dendritic cell-based cancer vaccine at its new production facility in Sawston, UK using advanced therapy medicinal products for the treatment of a glioblastoma case on compassion.
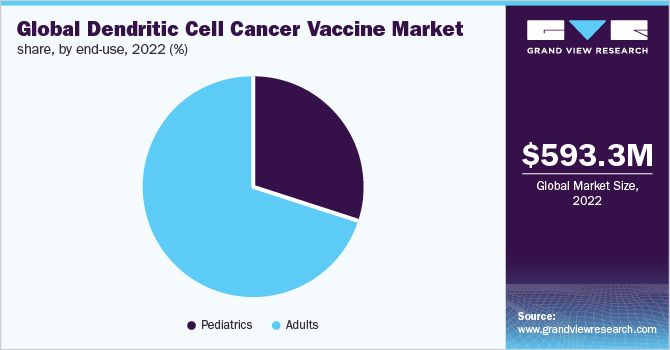
The pediatric vaccine segment is anticipated to grow significantly during the forecast period. Dendritic cell cancer vaccines are used to boost the immune mechanism of an individual at an early age, so as to eliminate the chances of tumor initiation and progression.
Regional Insights
North America leads the global dendritic cell cancer vaccine market in 2022. The growth of the dendritic cell cancer vaccine market in the region is mainly attributed to an unprecedented rise in the incidence of cancer cases and extensive research studies sponsored by academic research institutes and pharmaceutical giants. For instance, researchers at the Memorial Sloan Kettering Cancer Centre, U.S. have been researching mRNA vaccine technology and will be presenting results from the phase-1 trial at the annual meeting of the American Society of Clinical Oncology. mRNA vaccines are known to induce dendritic cells to make the neo-antigen proteins against carcinogenic cells.
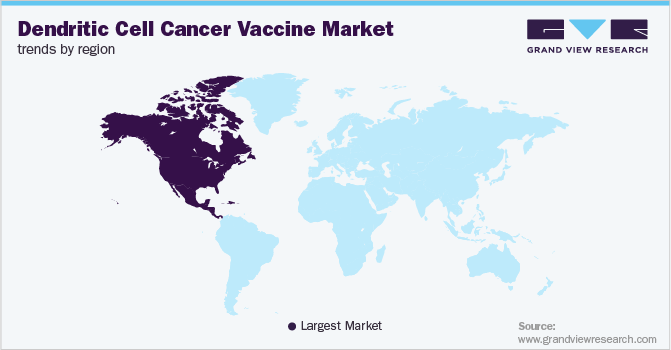
Europe is anticipated to show significant market growth in the coming years. This is attributed to the growing demand for cancer vaccines, the rising incidence of cancer, the rise in support for research studies from cancer societies across Europe, and emerging market players in the region positively impacts the growth of the dendritic cell cancer vaccine market.
Key Companies & Market Share Insights
Key players operating in the dendritic cell cancer vaccine market are undertaking various strategic initiatives such as partnership, collaboration, and new product launch to strengthen their market position. For instance, BioNTech signed into a collaboration with Medigene for the development of T-cell immunotherapies to target multiple solid tumor targets. The collaboration is intended for BioNTech to acquire Medigene's preclinical T-Cell Receptors (TCR) programs, which use Medigene’s switch-receptor technology for the development of TCR therapies and dendritic cell vaccines. Some of the prominent players in the global dendritic cell cancer vaccine market include:
-
GlaxoSmithKline plc,
-
3M Company
-
Activartis
-
Batavia Bioservices,
-
Argos Therapeutics
-
Sanpower Corporation
-
Elios Therapeutics
-
DanDrit Biotech
-
DCPrime
-
ImmunoCellular Therapeutics
Dendritic Cell Cancer Vaccine Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 652.52 million
Revenue forecast in 2030
USD 1.46 billion
Growth rate
CAGR of 12.18% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S., Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
3M company; GlaxoSmithKline plc; Activartis; Batavia Bioservices; Argos Therapeutics; Sanpower Corporation; Elios Therapeutics; DanDrit Biotech; DCPrime; ImmunoCellular Therapeutics
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Dendritic Cell Cancer Vaccine Market Segmentation
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global dendritic cell cancer vaccine market report based on product, end-use, and region
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
CreaVax
-
Sipuleucel-T (Provenge)
-
Other
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Pediatrics
-
Adults
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. North America dominated the dendritic cell cancer vaccine market with a share of 60.86% in 2022. This is attributable to the unprecedented rise in the incidence of cancer cases and extensive research studies sponsored by academic research institutes and pharmaceutical giants.
b. Some key players operating in the dendritic cell cancer vaccine market include 3M Company, GlaxoSmithKline plc, Activartis, Batavia Bioservices, Argos Therapeutics, Sanpower Corporation, Elios Therapeutics, DanDrit Biotech, DCPrime, and ImmunoCellular Therapeutics.
b. Key factors that are driving the market growth include The growth of the market is attributed to factors such as the increasing clinical trials activity related to cancer vaccines, growing demand for mRNA based vaccines along with evolving cancer therapeutics. Moreover, the growing demand for effective and personalized therapeutics is positively impacting the growth of the dendritic cell cancer vaccine market.
b. The global dendritic cell cancer vaccine market size was estimated at USD 593.30 million in 2022 and is expected to reach USD 652.52 million in 2023.
b. The global dendritic cell cancer vaccine market is expected to grow at a compound annual growth rate of 12.18% from 2023 to 2030 to reach USD 1.46 billion by 2030.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





