- Home
- »
- Pharmaceuticals
- »
-
Duchenne Muscular Dystrophy Drugs Market, Industry Report, 2023GVR Report cover
![Duchenne Muscular Dystrophy (DMD) Drugs Market Size, Share & Trends Report]()
Duchenne Muscular Dystrophy (DMD) Drugs Market Size, Share & Trends Analysis Report By Therapeutic Approach (Mutation Suppression, Exon Skipping, Steroid Therapy) And Segment Forecasts, 2018 - 2023
- Report ID: GVR-2-68038-423-9
- Number of Report Pages: 77
- Format: PDF, Horizon Databook
- Historical Range: 2015 - 2016
- Forecast Period: 2018 - 2024
- Industry: Healthcare
Industry Insights
The global Duchenne Muscular Dystrophy (DMD) drugs market size was valued at USD 365 million in 2017. It is anticipated to register a CAGR of 41.3% during the forecast period. The disease is the most common form of muscular dystrophy affecting 16 to 20 infants per 100,000 live births.
DMD is caused due to absence or deformity in the DMD gene that codes for dystrophin - a key protein for maintaining muscular integrity. The condition primarily affects males, as it is an X-linked genetic disorder. Females carrying the mutation in the dystrophin gene are usually asymptomatic or show mild symptoms.
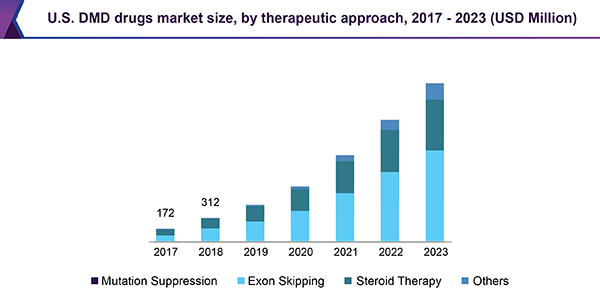
The market is projected to exhibit strong growth through the forecast period. The growth can be majorly attributed to factors such as the emergence of mutation-specific therapies, rising incidence of the condition, and improved diagnostics. Government initiatives supporting target-specific treatments and favorable reimbursement policies are expected to stoke the growth of the market. Furthermore, growing acceptance of targeted therapies, such as Exondys51, Translarna, and Emflaza, is likely to boost the therapeutic space.
The high cost of therapeutics remains an impediment to the growth of the DMD drugs landscape. However, affordable healthcare measures are being adopted by governments across all major regions, impacting the pricing strategies of companies as well as reimbursement scenario. Rising cost consciousness is poised to hinder premium pricing opportunities for upcoming DMD drugs.
Stringent regulatory approvals and lack of standardized procedures to determine the clinical efficacy of drugs remain significant challenges to overcome. However, increasing treatment options for underserved categories, such as infants and nonambulant patients, is estimated to augur well for the market in the foreseeable future.
Therapeutic Approach Insights
On the basis of the therapeutic approach, the market is divided into mutation suppression, exon skipping, and steroid therapy. An estimated 15.0% of all DMD cases result from nonsense mutations, leading to a truncated protein. Drugs such as ataluren (Translarna) bypass nonsense mutations. Mutation-suppressive drugs tend to prolong ambulation and have fewer adverse effects than steroids.
The exon-skipping approach is anticipated to register the steepest growth among all therapeutic methods. Nearly 50.0% and 90.0% of patients suffering from DMD mutations can benefit from single-exon skipping and multi-exon skipping, respectively. Sarepta’s Exondys51 is currently the only approved exon-skipping drug for DMD. Increasing adoption of this drug class and the launch of pipeline drugs are expected to fuel growth prospects.
Corticosteroids are the only class of drugs with proven efficacy in improving muscle strength in the case of DMD. Corticosteroids, such as deflazacort (Emflaza), have demonstrated delayed nonambulation; however, adverse effects of steroid therapy impedes their adoption.
Country Insights
Among the seven major markets, the U.S. dominated the DMD drugs market in 2017. The U.S. is expected to continue leading the market until 2023. High incidence of DMD in the U.S., booming treatment rate, and anticipated launch of pipeline products are some of the factors propelling the market in the country.
The EU5 has limited options for DMD as Exondys51 and Emflaza have not been approved in the region. Translarna remains the only treatment for nonsense mutation DMD patients who are in ambulatory state and are 5 years or older. The drug received conditional approval in Europe in 2014 and was labeled as an orphan medicinal product by the European Medicines Agency.
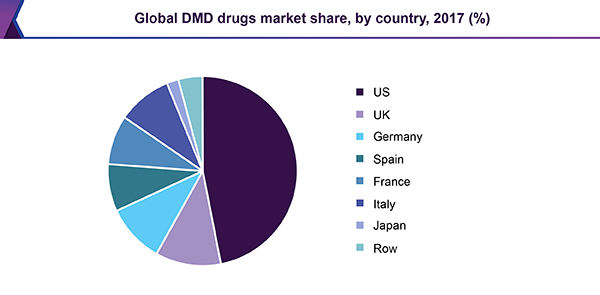
Japan is likely to witness the fastest growth among all the seven major markets during the forecast period. Currently, Prednisolone (corticosteroid) is the only approved drug for DMD. Japan’s 2014 guidelines for DMD recognized steroid therapy as the only treatment for muscle degeneration in DMD. However, the launch of pipeline drugs is poised to boost the development of other therapeutic approaches.
Duchenne Muscular Dystrophy (DMD) Drugs Market Share Insights
Some key players operating in the market are Sarepta, PTC, Santhera, Italfarmaco, and Catabasis. PTC and Sarepta led the market in 2017, by virtue of their products - Translarna, Emflaza, and Exondys51. Sarepta is projected to launch two late-stage pipeline products within the forecast period. In addition, Exondys51 sales are expected to cross USD 900 million. Sarepta is likely to lead the competitive landscape by 2023.
Raxone (by Santhera) and Givinostat (by Italfarmaco) are poised to be launched by 2023. Introduction of new products can cannibalize market share from PTC Therapeutics.
Report Scope
Attribute
Details
Base year for estimation
2017
Actual estimates/Historical data
2015 - 2016
Forecast period
2018 - 2023
Market representation
Revenue in USD Million & CAGR from 2017 to 2023
Country scope
U.S., U.K., Germany, Spain, Italy, France, Japan
Report coverage
Revenue forecast, company share, competitive landscape, growth factors and trends
15% free customization scope (equivalent to 5 analyst working days)
If you need specific information, which is not currently within the scope of the report, we will provide it to you as a part of customization
Segments Covered in the ReportThis report forecasts revenue growth at global, regional, & country levels and provides an analysis of industry trends in each of the sub-segments from 2017 to 2023. For the purpose of this study, Grand View Research has segmented the global Duchenne muscular dystrophy drugs market report based on therapeutic approach and country:
-
Therapeutic Approach Outlook (Revenue, USD Million, 2017 - 2023)
-
Mutation Suppression
-
Exon Skipping
-
Steroid Therapy
-
-
Country Outlook (Revenue, USD Million, 2017 - 2023)
-
The U.S.
-
The U.K.
-
Germany
-
Spain
-
Italy
-
France
-
Japan
-
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





