- Home
- »
- Pharmaceuticals
- »
-
Glioblastoma Multiforme Treatment Market Size Report, 2030GVR Report cover
![Glioblastoma Multiforme Treatment Market Size, Share & Trends Report]()
Glioblastoma Multiforme Treatment Market Size, Share & Trends Analysis Report By Treatment (Radiation Therapy, Immunotherapy), By Drug Class, By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68039-527-3
- Number of Report Pages: 110
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Report Overview
The global glioblastoma multiforme treatment market size was valued at USD 2.46 billion in 2022 and is anticipated to grow at a compound annual growth rate (CAGR) of 9.7% from 2023 to 2030. The growing prevalence of glioblastoma multiforme, increasing R&D expenditure, and favorable regulatory scenarios are among the factors anticipated to fuel market growth. The increasing incidence of brain tumors and the presence of a strong product pipeline is expected to act as a major driver for the glioblastoma multiforme (GBM) treatment market. As per the Global Cancer Observatory, in 2020, the incidence of brain & CNS cancers was 308,102, with the number of deaths standing at 251,329 worldwide. Brain cancer is a fatal form of cancer, with the WHO stating that over 245,000 cases of nervous system tumors and brain cancer were discovered in 2021.
Glioblastoma multiforme is the most common form of malignant tumor, accounting for up to 54% of gliomas and 16% of all primary brain cancers. The incidence of glioblastoma multiforme is higher in adults than in children, and the risk of developing glioblastoma multiforme increases with age. According to the American Association of Neurological Surgeons, in June 2021, glioblastoma accounted for 47.7% of all cases, impacting 3.21 per 100,000 people over 60 years of age and affecting men more likely than women.
The increasing approval for novel and combination therapies is expected to drive the market in the coming years. For instance, in June 2019, the U.S. FDA approved Pfizer’s ZIRABEV, a biosimilar for Avastin, for the treatment of recurrent glioblastoma, NSCLC, and colorectal cancer, among others. In January 2020, the company launched its product in the U.S. Moreover, in November 2019, the U.S. FDA accepted Samsung’s BLA application for its SB8 bevacizumab biosimilar candidate, increasing the potential to launch products in the coming years.
Tumor heterogeneity and variation in patient-to-patient treatment approach are expected to increase the demand for personalized treatments to manage glioblastoma multiforme. The approval of new treatments is expected to increase the life expectancy of patients. Moreover, a special designation granted to investigational drugs by the FDA is expected to expedite the approval process and commercialization of novel therapies. For instance, in July 2020, the U.S. FDA granted fast-track designation to Denovo Biopharma’s DB102 (enzastaurin) for the treatment of newly diagnosed glioblastoma patients.
Drug Class Insights
The other drug classes segment accounted for the largest revenue share in 2022. These include everolimus, corticosteroids, and 5-aminolevulinic acid (5-ALA). Corticosteroids are mainly prescribed to reduce peritumoral vasogenic edema for symptomatic benefits. Dexamethasone is mainly prescribed due to its lack of mineralocorticoid activity. Furthermore, corticosteroids may have immunosuppressive effects, emphasizing the opportunities to target immune responses for targeted therapy.
Bevacizumab is a targeted therapy approved for the treatment of recurrent and newly diagnosed GBM. In December 2017, Genentech received full approval from the U.S. FDA for bevacizumab (Avastin) for the treatment of adults with GBM. The medication is administered intravenously to stop the formation of new blood vessels that cut the blood supply and help reduce the tumor. Continuation in clinical studies to use bevacizumab with other therapies is expected to increase the usage of the drug for the management of GBM.
End-use Insights
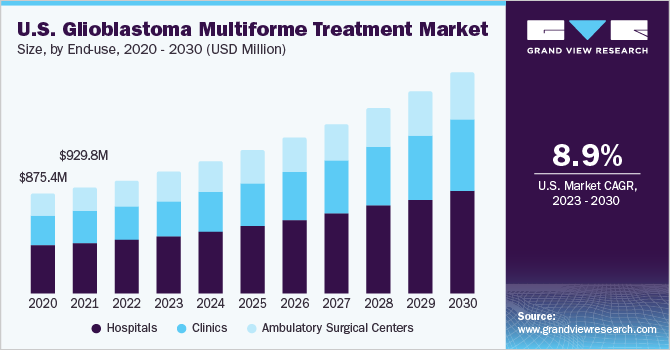
The hospital segment held the largest revenue share in the glioblastoma multiforme treatment market in 2022 and is anticipated to maintain its leading position during the forecast period. Patients prefer hospitals in terms of treatment accessibility and convenience. Moreover, the high number of surgeries performed to manage GBM and the complexity associated with the treatment procedure are anticipated to drive the market segment’s growth.
In July 2022, Diffusion Pharmaceuticals started a Phase II trial in glioblastoma multiforme patients that incorporated innovative imaging procedures to analyze tumor oxygenation. Many companies aim to develop and approve new drugs for treating neurology disease patients, including those suffering from glioblastoma and brain stroke. For instance, in July 2020, Denovo Biopharma’s DB102 (enzastaurin) fast-track designation was approved by the U.S. Food and Drug Administration (FDA) for treating newly identified glioblastoma patients.
The clinics segment is expected to expand at the fastest CAGR over the forecast period. Increasing adoption of noninvasive therapies, such as radiation therapy and tumor-treating field therapy, is anticipated to drive the segment. Increasing adoption of noninvasive and patient-oriented treatments, such as external beam radiation therapy and tumor-treating field therapy, is poised to boost industry growth. For instance, Johns Hopkins Medicine offers radiation therapy and tumor-treating field therapy, among others, for the treatment of brain cancer.
In August 2022, the United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA) approved Northwest Biotherapeutics’ Pediatric Investigation Plan (PIP), which was essential for the approval of new medicines such as DCVax-L, required by adult patients. According to a National Library of Medicine study published in November 2021, Glioblastoma Multiforme (GBM) has a low survival rate overall and a high frequency rate due to its aggressive nature. Radiation & chemotherapy are considered important and primary treatments after surgical resection.
On the other hand, the ambulatory surgical center segment is expected to witness lucrative growth over the forecast period. An increasing number of complex surgeries performed in outpatient settings may increase brain tumor resections at ambulatory surgical centers, thus driving its demand in the coming years.
Treatment Insights
The radiation therapy segment dominated the market with a revenue share of 37.0% in 2022, owing to improved survival rates, as radiation therapy is most often recommended as the first line of treatment or in combination with chemotherapy and surgery. It is also effective and highly recommended for brain tumors in case of a recurrence. In April 2020, a study on "Glioblastoma: Pathogenesis and Current Status of Chemotherapy and Other Novel Treatments" reported the availability of chemotherapeutic drugs such as temozolomide, Carmustine, and bevacizumab for effective treatment therapies.
The use of temozolomide, along with radiotherapy, makes the tumor more sensitive to radiation. This combination is more effective than radiation without temozolomide. In February 2023, the research team of Yale and The University of Connecticut discovered a nanoparticle-based treatment that helps cure multiple culprits in glioblastoma, which is the most dangerous form of brain cancer and can cause death. In September 2022, the American Society for Radiation Oncology (ASTRO) announced clinical guidelines to be followed for radiation therapy for treating patients with IDH-mutant grade 2 and 3 diffuse glioma, such as astrocytoma and oligodendroglioma.
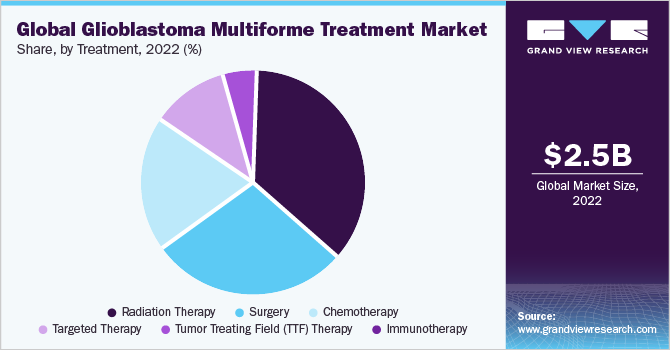
Surgery is the most common treatment for GBM, followed by radiation therapy and chemotherapy. This treatment plan is accepted as the standard of care. On the other hand, the increasing use of targeted therapy to manage GBM is expected to fuel segment growth, with this form expected to witness the fastest CAGR of 13.0% during the forecast period. Rising research activities to develop novel targeted therapies for the disease are also anticipated to boost segment expansion.
The Tumor Treating Field (TTF) therapy segment is also likely to show a strong growth in demand over the coming years. For instance, in July 2021, QV Bioelectronics declared to fund and support the development of its innovative GRACE implant, which helps to prevent cancer and tumor growth through electric therapy. QV Bioelectronics is a medical device start-up that develops a first-of-its-kind electric field therapy implant for glioblastoma multiforme.
Regional Insights
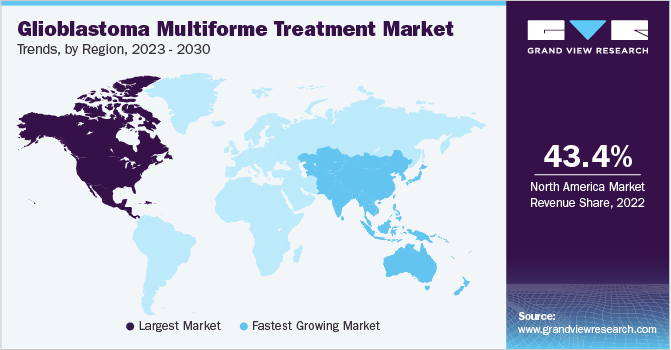
North America accounted for the largest share of 43.4% in 2022. Government support for the development of the healthcare sector, high awareness about rare disorders, easy accessibility to quality medical facilities, and favorable reimbursement policies are among the key factors responsible for the regional market growth. According to the American Cancer Society, an estimated 25,050 adults in the United States (14,170 men and 10,880 women) are likely to suffer from primary brain and spinal cord cancers in 2022.
About 85-90% of all primary CNS tumors occur in the brain. Moreover, 4,170 children under the age of 15 were diagnosed with brain or CNS tumors in the United States in 2022. According to Frontiers of Immunology’s (U.S.) study in March 2021, in clinical studies regarding glioblastoma multiforme, results for chemotherapy of antibodies, including PD-1/PD-11 with radiation therapy and chemotherapy, come out to be better and more promising than monotherapy with inhibitors such as PD-1/PD-11, which affects at a limited level.
Asia Pacific is expected to register the fastest growth rate of 11.1% over the forecast period, owing to various factors such as the entry of generics of temozolomide in the market, improving economy, rising geriatric population, and growing investments in the healthcare sector. The presence of large unmet needs in the region, growing awareness about GBM, and a robust product pipeline (molecules that can bypass the blood-brain barrier) are among the key factors contributing to regional market growth.
Key Companies & Market Share Insights
Key players in the GBM treatment market are focusing on R&D to develop advanced products to gain a competitive edge. Major players are engaged in mergers & acquisitions and licensing & partnerships to strengthen their product portfolio, expand their manufacturing capacities, and provide competitive differentiation. For instance, in August 2022, Aesther Healthcare Acquisition Corp. announced a merger agreement with Ocean Biomedical Inc., which is a next-generation biopharma company listed in NASDAQ. This merger is likely to help ease access to first-in-class vaccines and drugs.
In April 2021, Lineage Cell Therapeutics entered into a licensing agreement with Immunomic Therapeutics to develop a novel oncology candidate by leveraging the former’s cancer immunotherapy platform, allogeneic VAC, to treat glioblastoma multiforme. Under the agreement, an upfront payment of USD 2 million will be completed to Lineage, followed by USD 67 million as commercial milestones. Some prominent players in the global glioblastoma multiforme treatment market include:
-
Merck & Co., Inc.
-
Amgen, Inc.
-
F. Hoffmann-La Roche Ltd.
-
Pfizer Inc.
-
Teva Pharmaceutical Industries Ltd.
-
Sun Pharmaceutical Industries Ltd.
-
Arbor Pharmaceuticals, LLC
-
Amneal Pharmaceuticals
-
Karyopharm Therapeutics, Inc.
-
Sumitomo Dainippon Pharma Oncology, Inc. (Boston Biomedical, Inc.)
Glioblastoma Multiforme Treatment Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 2.67 billion
Revenue forecast in 2030
USD 5.10 billion
Growth Rate
CAGR of 9.7% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Treatment, drug class, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Key companies profiled
Merck & Co., Inc.; Amgen, Inc.; F. Hoffmann-La Roche Ltd.; Pfizer Inc.; Teva Pharmaceutical Industries Ltd.; Sun Pharmaceutical Industries Ltd.; Arbor Pharmaceuticals, LLC; Amneal Pharmaceuticals; Karyopharm Therapeutics, Inc.; Sumitomo Dainippon Pharma Oncology, Inc. (Boston Biomedical, Inc.)
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
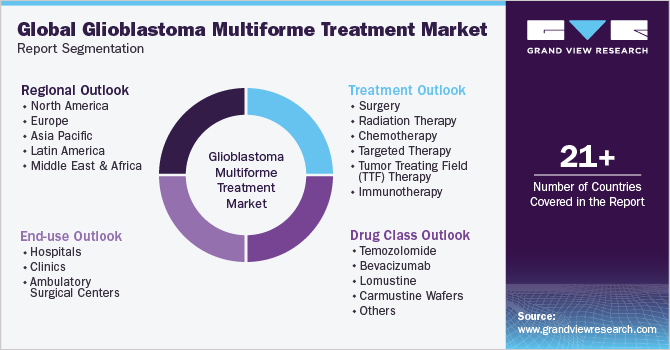
Global Glioblastoma Multiforme Treatment Market Report Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global glioblastoma multiforme treatment market report on the basis of treatment, drug class, end-use, and region:
-
Treatment Outlook (Revenue, USD Million, 2018 - 2030)
-
Surgery
-
Radiation Therapy
-
Chemotherapy
-
Targeted Therapy
-
Tumor Treating Field (TTF) Therapy
-
Immunotherapy
-
-
Drug Class Outlook (Revenue, USD Million, 2018 - 2030)
-
Temozolomide
-
Bevacizumab
-
Lomustine
-
Carmustine Wafers
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Clinics
-
Ambulatory Surgical Centers
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Norway
-
Denmark
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global glioblastoma multiforme treatment market size was estimated at USD 2.46 billion in 2022 and is expected to reach USD 2.67 billion in 2023.
b. The global glioblastoma multiforme treatment market is expected to grow at a compound annual growth rate of 9.7% from 2023 to 2030 to reach USD 5.10 billion by 2030.
b. Radiation therapy dominated the GBM treatment market with a share of 37.02% in 2022. The market share can be attributed to high penetration and increased adoption rate of the therapy owing to the rising prevalence of the disease in the market.
b. Some key players operating in the glioblastoma multiforme treatment market include Merck & Co., Inc., Amgen, Inc., and F. Hoffmann-La Roche Ltd.
b. Key factors that are driving the GBM treatment market growth include the growing prevalence of glioblastoma multiforme, increasing R&D, favorable regulatory scenario, and the presence of a strong pipeline.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





