- Home
- »
- Medical Devices
- »
-
Intraoperative Neuromonitoring Market Size Report, 2030GVR Report cover
![Intraoperative Neuromonitoring Market Size, Share & Trends Report]()
Intraoperative Neuromonitoring Market Size, Share & Trends Analysis Report By Product (Spinal Cord Stimulators, Deep Brain Stimulators), By Source (Parkinson’s Disease, Chronic Pain), By Type (Instruments, Consumables), By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-1-68038-767-4
- Number of Report Pages: 120
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
Market Size & Trends
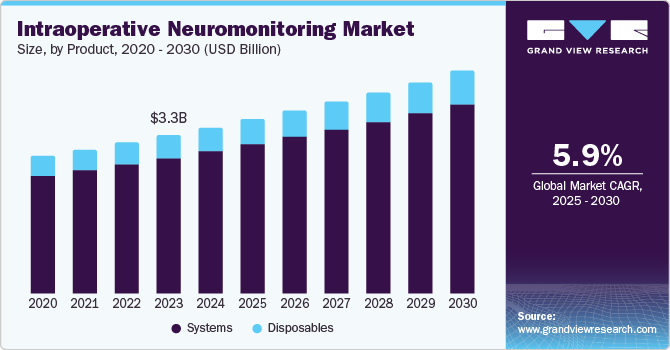
The global intraoperative neuromonitoring market size was estimated at USD 3.49 billion in 2024 and is anticipated to grow at a CAGR of 5.97% over the forecast period. The increasing incidence of chronic pain, neurological disorders, and mental health conditions is expected to drive the demand for effective and innovative treatment solutions. According to the Global Stroke Fact Sheet 2022 published by the World Stroke Organization (WSO), more than 12.2 million new strokes occur annually, and approximately 25% of the world's population over the age of 25 will experience a stroke in their lifetime. In addition, technological advancements, including creating more advanced and minimally invasive intraoperative neuromonitoring, further fuel market growth.
Moreover, there is a growing emphasis on patient education, comprehensive evaluation in recovery rooms for non-ICU hospitalizations, earlier mobilization, effective post-discharge communication, increased adoption of minimally invasive procedures, and prompt post-imaging assessments. These factors are projected to create substantial market growth opportunities in the coming years by enhancing patient care and optimizing post-operative management strategies. According to the CDC, adults aged 65 years and above are more likely to suffer from diabetes, COPD, heart disease, cancer, neurological problems, and other chronic illnesses. According to a Nature Reviews Neurology article published in September 2022, Alzheimer’s disease affects one out of every 10 people over the age of 65, and its prevalence rises with age. Patients suffering from such illnesses require both emergency and non-emergency hospital treatments, which is projected to drive market growth in the near future.
Monitoring the nervous system's health during spinal surgeries has been a major component of manufacturers' product differentiation strategy. With the evolution of IONM technology and techniques, the demand for neuromonitoring from surgeons and hospitals has increased over time. This is because of the availability of proprietary software from manufacturers and ease of operation. Intraoperative neuromonitoring devices enable surgeons to make key decisions in real-time, making surgeries safer, faster, and more repeatable, resulting in better patient outcomes and driving industry growth.
Moreover, the development of advanced devices, such as surgical robots, surgical microscopes, neurosurgery devices, and ophthalmic surgical devices has reduced direct human interference in surgeries, which is also anticipated to support market growth in the near future. For instance, in October 2020, Medtronic received 510(k) clearance from the U.S. FDA for the NIM Vital nerve monitoring system, which allows physicians to monitor, detect, and confirm nerve function to assist in reducing the risk of nerve damage during neck & head surgeries. The NIM Vital system utilizes patented technology to deliver precise intraoperative nerve status data that influences surgical strategies, improves operative efficiency & precision, and helps patients maintain their quality of life. Such developments are expected to impel growth in the near future.
Increasing government initiatives and growing awareness about products and services are further expected to boost industry growth. For instance, Accurate Neuromonitoring provided IONM in Africa during the 2019 Weill Cornell Tanzania Neurosurgery Mission. The mission trip raised awareness about the need for IONM in difficult brain & spine procedures and demonstrated the need for it. Similar advances in the market are expected to boost the demand for intraoperative neuromonitoring in the projection period.
Market Concentration & Characteristics
The market growth stage is moderate, and the pace of growth is accelerating. The intraoperative neuromonitoring market is characterized by a high degree of growth due to increasing demand for minimally invasive/noninvasive procedures, and advanced intraoperative neuromonitoring devices propels scientific advancements and encourage collaboration between researchers, medical professionals, and technology developers.
The intraoperative neuromonitoring market is experiencing high levels of innovation driven by continuous advancements in medical technologies. These innovations are improving the precision, reliability, and efficiency of neuromonitoring systems, making surgeries safer, and reducing the risk of neurological damage.

Regulatory bodies play a critical role in shaping the growth of the IONM market. Compliance with safety and performance standards is essential for product approvals. As regulations evolve to ensure better patient outcomes, companies are focusing on meeting these stringent requirements to maintain market access and build trust with healthcare providers. For instance, in February 2023, the FDA approved Medasense Biometrics Ltd.'s PMD-200, featuring the Nociception Level (NOL) Index monitoring system, for market use. This system assists healthcare professionals in evaluating patients' physiological responses to painful stimuli while under anesthesia and receiving opioid medications. The PMD-200 is intended to serve as a complementary tool, helping clinicians adjust opioid-based analgesics more precisely during anesthesia management.
The intraoperative neuromonitoring market is poised to experience robust mergers and acquisitions (M&A) activity as companies look to expand their product portfolios and strengthen their competitive positioning. For instance, in January 2023, Natus Medical Incorporated, a provider of medical device solutions for central nervous system and sensory system disorders, acquired Micromed Holding SAS, a provider of neurophysiology solutions. This strategic acquisition is estimated to enhance the product portfolio of Natus Medical Incorporated, further expanding its offerings in neurophysiology diagnostics and treatments.
Emerging substitutes and alternative monitoring technologies present both challenges and opportunities for the IONM market. Companies must continuously innovate to retain their market share and demonstrate the unique advantages of their offerings compared to these alternatives.
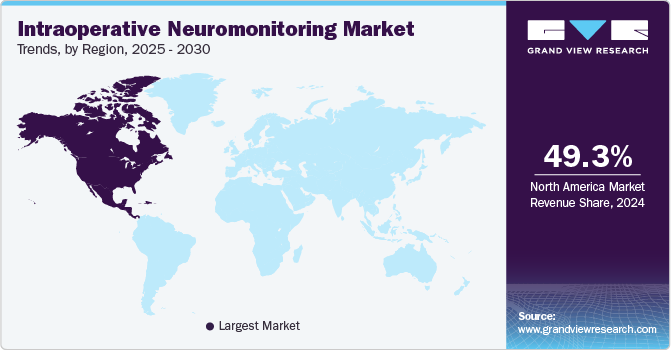
The global IONM market is expanding across various regions, driven by increased adoption of advanced monitoring techniques in surgeries. While mature markets in North America and Europe continue to dominate, emerging markets in Asia Pacific, Latin America, and the Middle East are showing considerable growth potential due to rising healthcare investments and greater awareness of patient safety.
Product Insights
The systemsegment held the largest market share of around 85.53% in 2024. The systems, including tools such as electromyography (EMG) monitors, electroencephalography (EEG) systems, and nerve stimulators, play a critical role in monitoring nerve function during complex surgeries. Their adoption is fueled by technological advancements that enhance surgical precision and minimize the risk of nerve damage. With a number of hospitals seeking to improve patient safety and surgical outcomes, the demand for these systems has been steadily growing. In addition, new innovations such as AI-powered systems are becoming more common, allowing real-time decision-making during surgeries, further solidifying the importance of this segment.
The disposables segment is expected to grow at the highest CAGR of 6.27% during the forecast period. This category covers consumables such as electrodes, sensors, and probes, which are essential for maintaining hygiene and preventing infections. Increased awareness around infection control, along with heightened safety protocols in operating rooms, is driving the demand for single-use products. In addition, the rise in complex spinal, neurosurgical, and orthopedic surgeries is increasing the need for specialized disposable components. Healthcare providers also prefer these products due to their ease of use and compliance with regulatory standards, paving the way for consistent market growth.
Source Insights
Insourced held the largest market share of around 52.72% in 2024. This can be attributed to The increase in the frequency of medical procedures and the need to monitor vital signs during these interventions, which have led to a growing adoption of intraoperative neuromonitoring (IONM) in hospitals. Many facilities are now training technologists to oversee these procedures, working closely with neurologists and physiologists. While there are currently no formal guidelines for the use of neuromodulation devices, their application is expected to become more widespread as demand rises. This approach allows healthcare facilities to maintain greater control over the quality and consistency of care provided. By having dedicated staff who are familiar with the specific needs and protocols of their institution, surgical teams can ensure seamless integration of neuromonitoring into their practices, ultimately enhancing patient safety and outcomes.
The outsourced segment is expected to grow at the highest CAGR of 8.13% during the forecast period. Owing to features such as cost-effectiveness and the availability of specialized service providers. Continuous advancements and modifications in IONM technology are enhancing its capabilities. Outsourcing these services allows hospitals to avoid the financial burden associated with purchasing and maintaining expensive equipment. In addition, training neurophysiologists can be both costly and time-consuming, as they must stay updated with the latest technologies. These factors are driving a notable shift toward outsourcing IONM procedures. Furthermore, by outsourcing, surgical teams can focus on their core responsibilities while relying on qualified personnel to manage neuromonitoring. This arrangement not only helps in optimizing resources but also ensures that patients receive high-quality care from experienced professionals in the field.
Type Insights
The intermittent monitoring segment dominated the market in 2024. This significant share indicates that hospitals and surgical centers still prefer cost-effective solutions for routine surgeries, where continuous oversight may not be essential. Intermittent monitoring provides clinicians with periodic updates on critical physiological parameters, allowing for sufficient interventions without incurring the higher costs of continuous monitoring systems. The preference for this approach is also driven by ease of implementation, especially in regions where healthcare facilities aim to balance efficiency with resource management. In addition, for surgeries of lower complexity, intermittent monitoring is sufficient to ensure patient safety, contributing to its sustained market leadership.
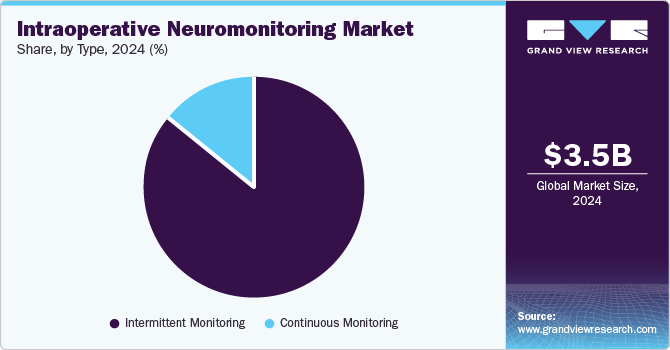
The continuous monitoring segment is expected to grow at the highest CAGR during the forecast period. This growth can be attributed to rising demand for real-time monitoring during high-risk or complex surgeries, such as neurosurgical and cardiovascular procedures, where immediate detection of complications is critical. Continuous monitoring systems offer a more comprehensive and dynamic view of the patient’s physiological state, enhancing the precision and safety of surgical interventions. Moreover, technological advancements, including the integration of artificial intelligence and remote monitoring capabilities, are further boosting the adoption of continuous monitoring solutions. As healthcare providers strive to improve patient outcomes, especially in tertiary care centers and advanced surgical units, this segment is expected to witness sustained growth, expanding its role in the overall intraoperative monitoring landscape.
Regional Insights
North America dominated the intraoperative neuromonitoring market with a share of 49.35% in 2024; favorable reimbursement policies and continuous innovation by key players further support the region's market growth. Moreover, advancements in technology, such as the development of portable IONM devices and improved monitoring techniques, are making it easier for healthcare providers to incorporate these systems into their practices. Training programs for technologists and surgeons are also expanding, ensuring that medical professionals are well-equipped to utilize IONM effectively. The U.S. remains the primary contributor, with Canada also playing a significant role through growing investments in healthcare technologies.
U.S. Intraoperative Neuromonitoring Market Trends
The intraoperative neuromonitoring market in the U.S. held a significant share of the market in 2024. Advanced healthcare infrastructure, a high number of surgical procedures, and increasing adoption of neuromonitoring technologies. Companies in the U.S. are focused on offering real-time monitoring solutions to minimize postoperative complications and improve patient outcomes, coupled with the rising neurovascular disorders. As per the National Institutes of Health, in January 2022, in the U.S., every year, around 795,000 people in the country have a stroke, causing 137,000 deaths. Thus, an increasing number of neurological disorder cases are expected to drive the market in this country. Furthermore, the presence of major industry players, increasing surgical procedures, and growing awareness about intraoperative neuromonitoring’s benefits are driving the market’s growth.
The intraoperative neuromonitoring market in Canada held a significant share of the market in 2024. Growing investments in healthcare and the rising adoption of neuromonitoring systems across hospitals are supporting market expansion.
Europe Intraoperative Neuromonitoring Market Trends
The intraoperative neuromonitoring market in Europe is anticipated to grow fastest with a CAGR of 6.38% during the forecast period. Driven by technological advancements and rising demand for precision surgeries. The UK, in particular, is expected to show notable growth due to increased healthcare spending and the growing focus on patient safety in surgical environments. In March 2023, Technomed Europe, known for Neurosign nerve monitoring products, was at S.K.V.T. Variscopic’s Clinical Career Event at the Technical University of Delft. Before this, they participated in the Wervingsdagen, the most significant technical career expo in the Netherlands, at the Technical University of Eindhoven.
UK intraoperative neuromonitoring market in is likely to show significant growth. The incidence of thyroid and parathyroid disorders in the UK is on the rise. Conditions such as thyroid cancer, hyperthyroidism, and primary hyperparathyroidism are becoming increasingly common. With this growing prevalence, there is an increasing demand for advanced surgical techniques and technologies that can improve outcomes and minimize the risk of complications. According to the University of Aberdeen, as of May 2023, one in every 20 people in the UK has a thyroid issue, with women being six times more likely to be affected than men. These trends are anticipated to drive growth in the healthcare market.
The intraoperative neuromonitoring market in Germany is likely to show significant growth. The healthcare system is adopting cutting-edge technologies such as intraoperative neuromonitoring (IONM) and fluorescent imaging systems. These innovations enhance surgical precision by better visualizing the thyroid and parathyroid glands, leading to more accurate resections and a reduced risk of hypoparathyroidism. Growing awareness among surgeons and increased training in the use of IONM systems are encouraging their adoption in thyroid and parathyroid surgeries. In addition, specialized workshops and medical conferences are playing a crucial role in educating healthcare professionals about these advanced tools.
Asia Pacific Intraoperative Neuromonitoring Market Trends
The intraoperative neuromonitoring market in Asia Pacific is experiencing rapid growth fueled by improving healthcare infrastructure and rising awareness about neuromonitoring technology. China is emerging as a lucrative market within the region, demonstrating strong growth potential due to increased government support and rising surgical volume. Furthermore, the presence of several notable players such as Medtronic, Natus Medical Incorporated, NuVasive, Inc., and Accurate Monitoring in China and Japan could potentially boost market growth.
The intraoperative neuromonitoring market in China is growing at a lucrative growth rate. The growing geriatric population, along with the increasing incidence of traumatic brain injuries, is expected to drive the regional market over the coming years. According to the Chinese Census estimates, the elderly population in the country is expected to further increase to 300 million by 2025 and cross 400 million by 2033.
Latin America Intraoperative Neuromonitoring Market Trends
The intraoperative neuromonitoring market in Latin America is experiencing significant growth. The growth is supported by expanding healthcare services and greater adoption of advanced medical technologies. Countries in the region are focusing on enhancing surgical outcomes, further driving demand for IONM solutions.
Middle East and Africa Intraoperative Neuromonitoring Market Trends
The intraoperative neuromonitoring market in MEA is experiencing growth driven by healthcare modernization efforts and the adoption of advanced surgical practices. Saudi Arabia, in particular, is driving market expansion, backed by increased healthcare investments and government initiatives aimed at improving patient care standards.
Saudi Arabia intraoperative neuromonitoring market is driven by the increasing prevalence of neurological disorders, and complex surgeries necessitate enhanced monitoring techniques to ensure patient safety and optimal outcomes. Additionally, the growing awareness among healthcare professionals about the benefits of intraoperative neuromonitoring is contributing to its adoption in surgical practices. Furthermore, advancements in technology, such as improved monitoring devices and techniques, are making it easier for hospitals and surgical centers to implement IONM. The support from the Saudi government for healthcare infrastructure development and investment in advanced medical technologies also plays a crucial role in fostering market growth.
Key Intraoperative Neuromonitoring Company Insights
The intensifying competition is leading to rapid technological advancements, and companies are constantly working to improve their products with a strong focus on research and development. Factors such as investments in R&D, compliance with regulatory policies, and technological advancements are constantly driving the introduction of novel techniques. In addition, market players are adopting strategies such as mergers & acquisitions, partnerships, product launches, and innovations to strengthen their foothold in the market. These advancements in the intraoperative neuromonitoring market are anticipated to boost the market growth over the forecast period.
Key Intraoperative Neuromonitoring Companies:
The following are the leading companies in the intraoperative neuromonitoring Market. These companies collectively hold the largest market share and dictate industry trends.
- Natus Medical Incorporated
- Accurate Monitoring
- Neuromonitoring Technologies
- Medtronic
- NuVasive, Inc.
- SpecialtyCare
- Neurosoft
- Ambu A/S
- Brainlab AG (Dr. Langer Medical GmbH)
- Welcony (Neurosign UK Ltd.)
Recent Developments
-
In July 2024, Soterix Medical received FDA 510(k) clearance for its MEGA-IOM system, designed to enhance intraoperative neurophysiologic monitoring (IOM). The system offers integrated monitoring of both central and peripheral nervous systems, using tools such as motor and sensory evoked potentials, EEG, and direct nerve stimulation to reduce postoperative risks and improve outcomes. Its modular design allows customization for various surgical needs, with options for portable or cart-based setups. Soterix also provides training and certification to support U.S. adoption.
-
In June 2024, The Neuro-IOM/Solo, an innovative 8-channel IONM system featuring a touchscreen, has been launched specifically for surgeon use. It offers monitoring through 8 bipolar channels, making it ideal for endocrine, maxillofacial, and ENT surgeries. The device simplifies the monitoring process significantly.
-
In September 2022, Brainlab announced the acquisition of Dr. Langer Medical GmbH, a German firm specializing in IONM solutions for surgery. This acquisition aims to enhance the digital surgery ecosystem, merging the innovation efforts of both companies. Dr. Langer Medical GmbH will operate independently within the Brainlab Group.
-
In January 2023, GE HealthCare announced its agreement to acquire Imactis, a company specializing in interventional radiology guidance systems. This acquisition aims to strengthen GE’s capabilities in precision healthcare by integrating Imactis' advanced CT-based navigation technology, which supports minimally invasive procedures. GE HealthCare intends to enhance access to these technologies, improving treatment precision and patient outcomes.
Intraoperative Neuromonitoring Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 3.66 billion
Revenue forecast in 2030
USD 4.66 billion
Growth rate
CAGR of 5.97% from 2025 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million and CAGR from 2025 to 2030
Segment scope
Product, source, type, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Report Coverage
Revenue, competitive landscape, growth factors, and trends
Key companies profiled
Natus Medical Incorporated; Accurate Monitoring; Neuromonitoring Technologies; Medtronic; NuVasive, Inc.; SpecialtyCare; Neurosoft; Ambu A/S; Brainlab AG (Dr. Langer Medical GmbH); Welcony (Neurosign UK Ltd.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Intraoperative Neuromonitoring Market Segmentation
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global intraoperative neuromonitoring market report on the basis of product, source, type, and region:
-
Product Outlook (Revenue USD Million, 2018 - 2030)
-
Systems
-
Disposables
-
EMG Tubes And Electrodes
-
Sticker Electrode
-
Stimulating Probes
-
-
-
Source Outlook (Revenue USD Million, 2018 - 2030)
-
Insourced
-
Outsourced
-
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Intermittent Monitoring
-
Continuous Monitoring
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Norway
-
Denmark
-
Sweden
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. Key factors that are driving the market growth include a rising number of complex surgeries, including spinal, neurosurgical, and orthopedic procedures, and increases the demand for IONM to minimize risks of nerve damage and ensure patient safety. Technological advancements in IONM, such as real-time remote monitoring and enhanced signal detection, have improved surgical outcomes and boosted its adoption.
b. The global intraoperative neuromonitoring market size was estimated at USD 3.49 billion in 2024 and is expected to reach USD 3.66 billion in 2025.
b. The global intraoperative neuromonitoring market is expected to grow at a compound annual growth rate of 5.97% from 2025 to 2030 to reach USD 4.66 billion by 2030.
b. North America dominated the intraoperative neuromonitoring market with a share of 49.35% in 2024. This is attributable to high surgical volume, robust healthcare infrastructure, and favorable reimbursement policies.
b. Some key players operating in the intraoperative neuromonitoring market include Natus Medical Incorporated, Accurate Monitoring, Neuromonitoring Technologies, Medtronic, NuVasive, Inc., SpecialtyCare, LLC, Neurosoft, Ambu A/S, Brainlab (Dr. Langer Medical GmbH), and Welcony (Neurosign UK Ltd.)
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





