- Home
- »
- Medical Devices
- »
-
Intrapartum Monitoring Devices Market Size Report, 2030GVR Report cover
![Intrapartum Monitoring Devices Market Size, Share & Trends Report]()
Intrapartum Monitoring Devices Market (2024 - 2030) Size, Share & Trends Analysis Report By Product (Monitors, Electrodes), By End Use (Hospitals, Maternity Centers), By Region, And Segment Forecasts
- Report ID: GVR-2-68038-308-9
- Number of Report Pages: 80
- Format: PDF
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Market Size & Trends
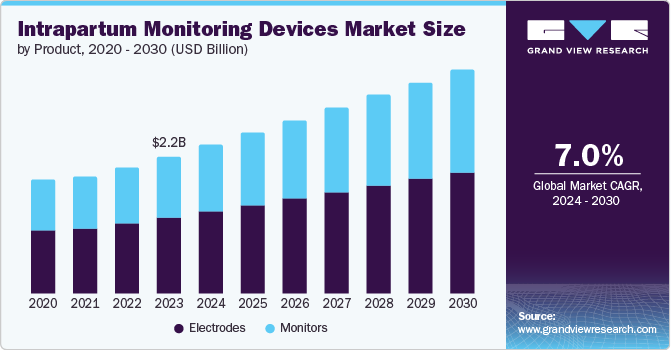
The global intrapartum monitoring devices market size was valued at USD 2.15 billion in 2023 and is projected to grow at a CAGR of 7.0% from 2024 to 2030. The rise in premature births, improved maternal care, and rapid expansion of healthcare infrastructure across the globe have triggered the need for intrapartum monitoring devices. In addition, technological advancements in non-invasive monitoring techniques and the development of portable devices have made these devices more accessible and user-friendly. Furthermore, government initiatives and increased funding for maternity and child health programs are anticipated to drive market growth.
Additionally, the global rise in high-risk pregnancies is a prominent factor in the market growth. Some conditions such as preeclampsia, gestational diabetes, and others need additional attention to ensure the safety of both the mother and the unborn child. Rising consciousness and health awareness campaigns regarding fetal and maternal care during the labor process have also contributed to the increasing popularity of these products. Hospitals and other healthcare facilities are adopting state-of-the-art monitoring systems to enhance patient care.
The enhanced use of artificial intelligence (AI) and machine learning (ML) in intrapartum monitoring devices is trending. Such technologies enable prediction and real-time data analysis that help to understand any adverse occurrences during the treatment course and notify the healthcare providers. The shifts in the healthcare sector are encouraging the use of monitoring devices for fetal care. With the increase in premature births in remote areas, the need for intrapartum monitoring devices is large. Furthermore, collaborations and partnerships between medical device manufacturers and healthcare institutions foster innovation and accelerate the development and adoption of cutting-edge monitoring technologies are anticipated to drive market growth.
Product Insights
Electrodes dominated the market with a share of 55.3% in 2023. Electrodes offer an accurate way of monitoring fetal heart rate compared to non-invasive methods. In addition, their widespread use in fetal heart rate monitoring systems is increasing due to concerns about fetal health during delivery. Furthermore, the rising prevalence of gestational diabetes in expecting mothers can affect fetal well-being and is anticipated to drive market growth.
Monitors are expected to register a CAGR of 7.5% during the forecast period due to the adoption of advanced monitoring technologies that provide real-time data on fetal and maternal health. In addition, increasing awareness and demand for comprehensive maternal and fetal care and an increasing trend towards continuous and remote monitoring are anticipated to drive market growth. Furthermore, innovation in wireless and non-invasive monitors, integrated into telehealth platforms make these devices increasingly popular in the healthcare sector.
End Use Insights
The hospital segment dominated the market in 2023 due to the high demand for advanced monitoring technologies and comprehensive maternal care services offered in these healthcare institutions. Additionally, dedicated maternity hospitals have experienced healthcare professionals dealing with delivery complications. Furthermore, rising cases of high-risk pregnancies and complications during labor calls for intrapartum monitoring in hospitals to ensure optimal outcomes for the mother and the fetus.
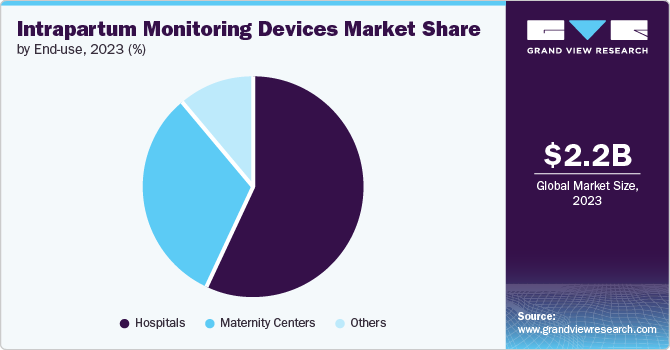
The maternity centers segment is projected to grow at a CAGR of 7.5% over the forecast period. This is due to the increasing demand for specialized maternal care and personalized delivery experiences in these centers. Additionally, increasing awareness and demand for dedicated maternity services coupled with advancements in intrapartum monitoring technologies, enhance maternity centers' appeal. Furthermore, the increasing use of outpatient home deliveries assisted by portable and user-friendly monitoring devices is anticipated to drive the segment growth.
Regional Insights
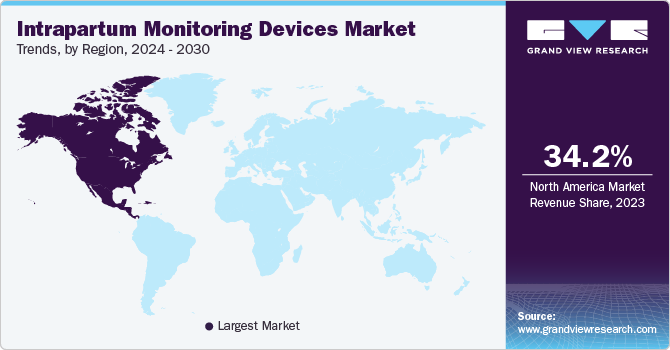
North America intrapartum monitoring devices dominated the global market in 2023. The region has a highly developed healthcare infrastructure and modern hospitals with cutting-edge monitoring equipment. Additionally, the increased consciousness for the quality of life and the health of the mother and fetus drives the need for these devices. Furthermore, a strong focus on technological advancements by key players in the regional market ensures a steady stream of innovative and reliable monitoring solutions.
U.S. Intrapartum Monitoring Devices Market Trends
The U.S. intrapartum monitoring devices market dominated North America with a share of 87.4% in 2023 due to the country's advanced healthcare infrastructure and high adoption of cutting-edge medical technologies. The increasing incidence of high-risk pregnancies and a strong focus on improving maternal and fetal health outcomes is anticipated to drive demand for these devices. Additionally, significant investments in healthcare and supportive government policies are further propelling the U.S. market growth.
Europe Intrapartum Monitoring Devices Market Trends
Europe intrapartum monitoring devices market remained substantial in 2023. This is due to European countries’ emphasis on improving healthcare standards and investments in advanced medical technologies. Additionally, the increasing pregnancy complications have bolstered the demand for robust intrapartum monitoring. Furthermore, government policies and programs to tackle issues related to declining birth rates in Europe are further anticipated to drive the regional market growth.
The UK intrapartum monitoring devices market is expected to grow in the coming years. The NHS has unveiled a three-year delivery plan that enhances maternity and neonatal services across England. This initiative focuses on improving care quality, ensuring safer births, and enhancing support for mothers and babies. It outlines strategic goals to modernize healthcare facilities and technological investments and prioritize patient safety in maternity and neonatal care. Furthermore, increased focus on reducing interventions during childbirth, while maintaining safety is anticipated to drive the adoption of precision monitoring.
Asia Pacific Intrapartum Monitoring Devices Market Trends
Asia Pacific intrapartum monitoring devices market is anticipated to witness significant growth. This is due to the availability of better healthcare facilities with enhanced care for mothers and childcare. Additionally, increasing consciousness among the population concerning maternal and fetal tracking, along with the growth of the childbirth rate across the region and the high incidence of risky pregnancies is anticipated to drive the market growth. Furthermore, the employment of advanced technologies in the development of these devices, and affordable monitoring solutions are further anticipated to propel the market growth.
The China intrapartum monitoring devices market held a substantial share in 2023 due to the high population and birth rate, which drives demand for effective maternal and fetal screening solutions. Additionally, the government's focus on improving healthcare policies and increased investment in advanced medical technology is anticipated to drive market growth.
Key Intrapartum Monitoring Devices Company Insights
Some key companies in the global intrapartum monitoring devicesmarket include Cardinal Health, MindChild Medical, Koninklijke Philips N.V., GE Healthcare, and others. Vendors in the market are focusing on increasing their customer base to gain a competitive edge in the industry. Therefore, key players are taking several strategic initiatives, such as mergers and acquisitions and partnerships with other major companies.
-
GE Healthcare is a global leader in medical technology. It focuses on improving healthcare through innovative devices and smart solutions. Its offerings range from imaging systems (MRI, CT scans) and ultrasound systems to patient monitoring devices and data analysis software.
Key Intrapartum Monitoring Devices Companies:
The following are the leading companies in the intrapartum monitoring deviceses market. These companies collectively hold the largest market share and dictate industry trends.
- Cardinal Health
- MindChild Medical
- Koninklijke Philips N.V.
- CooperSurgical, Inc
- GE Healthcare
- Analogic Corporation
- Olympus Corporation
- Medtronic plc
- Stryker
- Omron Corporation
Recent Developments
-
In June 2024, Stryker announced the expansion of its innovation and R&D capabilities with a new medical device testing facility. This state-of-the-art facility will enhance Stryker's ability to develop and rigorously test medical devices, ensuring higher quality and safety standards. The expansion underscores Stryker's commitment to advancing medical technology and improving patient care.
-
In April 2024, GE HealthCare received FDA clearance for its Portrait VSM, a wireless vital sign monitoring system that constantly monitors patient health. This new device integrates into GE's ecosystem of connected patient monitoring solutions, enhancing real-time data sharing and patient care. The approval highlights GE HealthCare's commitment to advancing medical technology and improving patient outcomes through innovative monitoring solutions.
Intrapartum Monitoring Devices Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 2.33 billion
Revenue forecast in 2030
USD 3.50 billion
Growth Rate
CAGR of 7.0% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, end use, and region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Cardinal Health; MindChild Medical; Koninklijke Philips N.V.; CooperSurgical; Inc; GE Healthcare; Analogic Corporation; Olympus Corporation; Medtronic plc; Stryker; Huntleigh Healthcare Limited
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Intrapartum Monitoring Devices Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global intrapartum monitoring devicesmarket report based on product, end use, and region.
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Monitors
-
Electrodes
-
Fetal Scalp Electrodes
-
Intrauterine Pressure Catheter
-
Transducer for FHR
-
Transducer for Uterine Contractions
-
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Maternity Centers
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










